Abstract
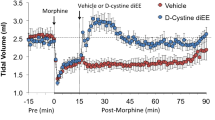
Dynorphins, such as dynorphin A(1–13) (Dyn A(1–13)), have been shown to enhance analgesia in morphine-tolerant animals, despite their very short half-life after intravenous administration. The potential use of dynorphins in humans is therefore of interest. This laboratory has recently evaluated the metabolic fate of stabilized dynorphin derivatives. This study was conducted to evaluate whether such stabilized derivatives, ie, [N-Met-Tyr1]-Dynorphin A(1–13) (N-MT Dyn A, stabilized at the N-terminal end) and [N-Met-Tyr1]-Dynorphin A(1–13) amide (N-MT Dyn A amide, stabilized at the C-and N-terminal ends), would enhance the antinociceptive activity of morphine not only after intravenous administration but also after subcutaneous and pulmonary delivery. Intravenous administration of N-MT Dyn A (5 μmol/kg) and N-MT Dyn A amide (5 μmol/kg) to morphine-tolerant rats resulted in significantly higher tail-flick latencies than those observed for the saline group. These effects could be observed for up to 2.0±0.1 hours after intravenous administration of N-MT Dyn A and for up to 3.4±1.4 hours for N-MT Dyn A amide. The time-averaged effects of both peptides were similar. After pulmonary delivery of the same dose, derivatives remained active. The duration of the effects after pulmonary administration of the amide was 4.4±2.5 hours while that of N-MT Dyn A was slightly shorter (2.8±0.9 hours). No effect was observed after subcutaneous administration of N-MT Dyn A. These results suggest that pulmonary delivery of stabilized dynorphin derivatives represents a possible alternative to intravenous administration.
Similar content being viewed by others
References
Goldstein A, Tachibana S, Lowney S, Hunkapiller M, Hood L. Dynorphin-(1–13), an extraordinarily potent opioid peptide.Proc Natl Acad Sci USA. 1979;76:6666–6670.
Friedman HJ, Jen M-F, Chang JK, Lee NM, Loh HH. Dynorphin: a possible modulatory peptide on morphine or b-endorphin analgesia in mouse.Eur J Pharmacol. 1981;69:357–360.
Walker JM, Katz RJ, Akil H. Behavioral effects of dynorphin1–13 in the mouse and rat: initial observations.Peptides. 1980;1:341–345.
Pentel PR, Wananukul W, Hooke LP, et al. Effects of high intravenous doses of dynorphin A (1–13) on tail flick latency and central nervous system histology in rats.Pharmacol Biochem Behav. 1995;51:387–390.
Tulunay FC, Jen M-F, Chang J-K, Loh HH, Lee NM. Possible regulatory role of dynorphin on morphine- and b-endorphin-induced analgesia.J Pharmacol Exp Ther. 1981;219:296–298.
Lee NM, Smith AP. Possible regulatory function of dynorphin and its clinical implications.Trends Pharmacol Sci. 1984;5:108–110.
Green PG, Lee NM. Dynorphin A-(1–13) attenuates withdrawal in morphine-dependent rats: effect of route of administration.Eur J Pharmacol. 1988;145:267–272.
Takemori AE, Loh HH, Lee NM. Suppression by dynorphin A-(1–13) of the expression of opiate withdrawal and tolerance in mice.Eur J Pharmacol. 1992;221:223–226.
Hooke LP, He L, Lee NM. Dynorphin A modulates acute and chronic opioid effects.J Pharmacol Exp Ther. 1995;273:292–297.
Aceto MD, Dewey WL, Chang JK, Lee NM. Dynorphin (1–13) effects in nontolerant and morphine-dependent rhesus monkeys.Eur J Pharmacol. 1982;83:139–142.
Wen HL, Ho WKK. Suppression of withdrawal symptoms by dynorphin in heroin addicts.Eur J Pharmacol. 1982;82:183–186.
Muller S, Hochhaus G. Metabolism of dynorphin, A 1–13 in human blood and plasma.Pharm Res. 1995;12:1165–1170.
Woo S, Garzon J, Sanchez-Blazquez P, Tulunay FC, Chang JK, Loh HH. Dynorphin (1–10)amide: a potent and selective analog of dynorphin(1–13).Life Sci. 1982;31:1817–1820.
Yoshino H, Nakazawa T, Arakawa Y, et al. Synthesis and structure-activity relationships of dynorphin A(1–8) amide.J Med Chem. 1990;33:206–212.
Yu J, Butelman RB, Woods JH, Chait BT, Kreek MJ. Dynorphin A(1–8) analog, E-2078, is stable in human and rhesus monkey blood.J Pharmacol Exp Ther. 1996;280:1147–1151.
Hooke LP, He L, Lee NM. [Des-Tyr1] Dynorphin A-(2–17) has naloxone-insensitive antinociceptive effect in the writhing assay.J Pharmacol Exp Ther. 1995;273:802–807.
Al-Fayoumi S, Brugos B, Arya V, et al. Identification of stable dynorphin derivatives for suppressing tolerance in morphine dependent rats.Pharm Res. 2004; In press.
Caudle RM, Isaac L. Intratechal dynorphin (1–13) results in irreversible loss of tail flick reflex in rats.Brain Res. 1987;435:1–6.
Laughlin TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent nociception by dynorphin.J Pharmacol Exp Ther. 2001;299:6–11.
D'Amour FE, Smith DCA. A method for determining loss of pain sensation.J Pharmacol Exp Ther. 1941;72:74–79.
Dewey WL, Harris LS. The tail-flick test. In: Neidle A, ed.Methods in Narcotics Research. New York: Marcel Dekker Inc; 1975:101–109.
Lohmann A, Smith F. Buprenorphine substitution ameliorates spontaneous withdrawal in fentanyl-dependent rat pups.Pediatr Res. 2001;49:50–55.
Harris LS, Pierson AK. Some narcotic antagonists in the benzomorphan series.J Pharmacol Exp Ther. 1964;143:141–148.
Hochhaus G, Derendorf H. Dose optimization based on pharmacokinetic/pharmacodynamic modeling. In: Derendorf H, Hochhaus G, eds.Handbook of Pharmacokinetic/Pharmacodynamic Correlations. New York: CRC; 1995:79–120.
Gambus PL, Schnider TW, Minto CF, et al. Pharmacokinetics of intravenous dynorphin A(1–13) in opioid-naive and opioid-treated human volunteers.Clin Pharmacol Ther. 1998;64:27–38.
Agu RU, Ugwoke MI, Armand M, Kinget R, Verbeke N. The lung as a route for systemic delivery of therapeutic proteins and peptides.Respir Res. 2001;2:198–209.
Cefalu WT, Rosenstock J, Blindra S. Inhaled insulin: a novel route for insulin delivery.Expert. Opin. Drugs. 2002;11:687–691.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published: December 28, 2004.
Rights and permissions
About this article
Cite this article
Brugos, B., Arya, V. & Hochhaus, G. Stabilized dynorphin derivatives for modulating antinociceptive activity in morphine tolerant rats: Effect of different routes of administration. AAPS J 6, 36 (2004). https://doi.org/10.1208/aapsj060436
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/aapsj060436