Abstract
Background
Ischemic stroke is a major cause of death and disability. Thrombolytic therapy is a standard treatment stroke nowadays for ischemic strokes up to 4.5 h from start of symptoms. Although arterial occlusion can be detected by digital subtraction angiography (DSA), magnetic resonance angiography (MRA), and computed tomography angiography (CTA), the question about thrombus composition and formation times still might not be replied. The use of susceptibility weighted imaging (SWI) for detecting thrombus in acute ischemic stroke is getting to be a strongly investigated field. SWI can show the thrombus as a hypointense susceptibility vessel sign (SVS) in the affected area.
Results
Ninety-seven of our patients showed thrombus in MRA study. M1 segment was the most affected MCA segment representing about 57.6%. SWI detected intra-arterial thrombus in 122 patients compared to 97 patients detected by MRA (P = 0.0002). All patients had positive susceptibility sign. 88.8% of patients with positive thrombus in SWI had solitary thrombus, and 11.2% has multiple thrombi; on the other hand, MRA fails to detect any distant thrombi. 81% of patients with abnormally prominent vessel sign (APVS) showed parenchymal changes in these areas. On the other hand, deep structures, namely caudate nucleus, internal capsule and lentiform nucleus, are the least affected areas. All patients with abnormally prominent vessel sign showed arterial occlusion, and only 9 patients with no APVS showed arterial occlusion (P = 0.0001).
Conclusion
SWI plays an important role in the detection of peripheral thrombi in patients with acute ischemic stroke. Both SWI and MRA might complement each other for visual detection of occluded vessel. We recommend implementation of SWI into routine acute stroke MRI protocols.
Similar content being viewed by others
Background
Ischemic stroke is a major cause of death and disability, and thrombolytic therapy is the only proven treatment for those patients within 3 or 4 h of symptom onset [1]. It has been proposed that the nature of the thrombus may affect recanalization, and red thrombus can be readily recanalized after thrombolytic therapy. [2,3,4]. Also, the site and size of thrombus could influence the treatment strategy [5, 6]. Although intra-vascular thrombus can be detected by the means of digital subtraction angiography (DSA), magnetic resonance angiography (MRA), and computed tomography angiography (CTA), questions about thrombus composition and time of formation could not yet be answered [6,7,8,9,10,11]. Susceptibility weighted imaging (SWI) is a magnetic resonance imaging (MRI) technique that enhances image contrast by using the susceptibility differences between tissues. The strength of SWI is in its ability to identify hemorrhage, calcium and nonheme iron by virtue of its susceptibility artifact. Susceptibility weighted imaging (SWI) has become a key MR sequence in pediatric neuroimaging. The use of susceptibility weighted imaging (SWI) in the visualization of thrombi in acute ischemic stroke has become of major interest [12,13,14]. In SWI, intravascular thrombus manifests as an abnormal dark signal known as a susceptibility vessel sign (SVS), with the classic appearance as a thick vessel containing dark signal. SVS indicates an increase in locally elevated deoxyhemoglobin from trapped red blood cells (erythrocytes) in blocked vessels. [4, 13, 15]. The presence of SVS promotes acute or subacute thrombus [16, 17] SVS can provide useful information about the site, multiplicity and structure of thrombus [18, 19]. SVS means arterial occlusion [20] and resolves upon arterial recanalization [14, 21]. Using the fact that thrombus synthesis is different and only the acute phase of thrombus can show SVS on SWI, we hypothesized that susceptibility not only reflects the structure of thrombus but also its time of formation also.
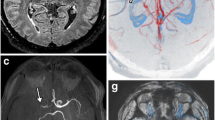
The aim of the current study is to assess the value of susceptibility weighted imaging in assessment of intra-arterial thrombus in correlation with MRA and diffusion weighted imaging in patients with acute ischemic infarction (Figs. 1, 2, 3, 4).
Methods
Study design and population
This retrospective study included 150 patients diagnosed as diagnosed stroke (age range from 30 to 70 years, mean 39.5 ± 10.4 years); study was approved by the Ethic Committee in our institution and was conducted in the time period from August 2019 to April 2021. All patients were referred during the first 24 h from the onset (mean time about 18 h).
Inclusion and exclusion criteria
Inclusion criteria
-
Acute stroke.
-
Stroke lasting > 1 h.
Exclusion criteria
General contraindications to MRI such as presence of any paramagnetic substances and claustrophobic patients.
MRI technique
Magnetic resonance imaging studies were performed on 1.5-T system magnet (Aera, Siemens Heath care, Siemens AG, Munchen, Germany). All patients underwent MRI scan using the following imaging sequences: axial fast field echo (FFE) T1, axial turbo spin-echo (TSE) T2, FLAIR, and DWI, in addition to the SWI sequence and MRA.
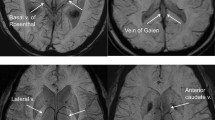
The SWI sequence was obtained with the following parameters: TR = 34 ms; TE = 24 ms; flip angle = 15°, slice thickness = 0.5 mm with 240 slices per slab, voxel size = 1.1 × 1.1 × 0.5 mm and matrix size = 208 × 173. The acquisition time was 7 min, 57 s. Post-processing included minimum intensity projection (minIP) images with 10 mm thickness to assess the venous collaterals and maximum intensity projection (MIP) to assess the cerebral vessels.
Image interpretation
Images were reviewed by a radiologist with 10-year experience in neuroradiology and was blinded to clinical data. Intra-arterial thrombus was detected using susceptibility vessel sign (SVS) which appeared as an abnormal low signal intensity within the artery which appeared larger than the size of vessel in contralateral side. The thrombus can be detected during the first week of attack and can be measured in the slice showing longest SVS sign. Findings were compared with MRA.
Statistical analysis
Statistical analysis was performed using the SPSS software for Windows v. 20 (SPSS Inc., Chicago, IL). For tests of significance (repeated measures ANOVA, Cochran’s Q tests, Kappa statistics and ROC curve analysis), P-values less than 0.05 (5%) were considered to be statistically significant.
Results
Ninety-seven of patients showed thrombus in MRA. M1 segment was the most affected MCA segment representing about 57.6%. M5 segment was the least affected segment. SWI detected intra-arterial thrombus in 122 patients compared to 97 patients detected by MRA (P = 0.0002). All patients had positive susceptibility sign (Table 1).
88.8% of patients with positive thrombus in SWI had solitary thrombus, and 11.2% has multiple thrombi; on the other hand, MRA fails to detect any distant thrombi (Table 2).
M4, M5 and M6 were the most affected segments; 81% of patients with abnormally prominent vessel sign (APVS) showed parenchymal changes in these areas. On the other hand, deep structures, namely caudate nucleus, internal capsule and lentiform nucleus, are the least affected areas. All patients with abnormally prominent vessel sign showed arterial occlusion, and only 9 patients with no APVS show arterial occlusion (P = 0.0001) (Table 3).
A total of 102 of our patients showed mismatch between SWI and DWI representing about 68%, and 48 patients have negative mismatch representing 32% (Table 4).
Discussion
SWI plays an important role in acute cerebral ischemia by detecting the area at risk and area of hemorrhagic transformation; in addition, SWI has an important role in assessment of intra-arterial thrombus regarding the site, length of the thrombus and number of the thrombi depending upon the susceptibility vessel sign, on SWI and GRE T2* sequences; this sign is detected by its hypointensity within the lumen of the occluded artery which appears larger in diameter compared to the contralateral normal vessel.
Susceptibility weighted imaging (SWI), an increasingly used MR sequence in recent years with a high sensitivity effect, can better detect vascular anomalies such as the DVA and has been shown to be more sensitive than conventional T2* sequence in the detection of vascular structures [10]. SWI can demonstrate the whole vascular structure well and typically has a low signal intensity due to the blood oxygen level-dependent (BOLD) effect in the dilated medullary and draining veins [22]. Compared to T2 * gradient echo (GRE), SWI has high sensitivity and good contrast resolution for detection of thromboembolism in the anterior and posterior circulations [23]. During the pioneering era of CT, it was shown that the hyperdense middle cerebral artery (MCA) and insular points represent the presence of thromboembolism; However, the reliability of these signs depends on the Hounsfield attenuation of thromboembolism, which is influenced by fibrin content [23]. A major disadvantage of non-contrast TOF angiography is its insensitivity toward slow flow or slow in-plane flow.
60% of patients in the current study showed left cerebral ischemia; in contrast to this, various articles exhibited different findings to our study.
In the current study, M1 segment was the most affected middle cerebral artery segment in MRA, 33 patients showed thrombus in left M1, and 12 patients showed thrombus in right M1 segment representing about 60% of all patients detected in MRA. On the other hand in a study done by park M-G et al. [27], M5 segment was the least affected segment in MRA and SWI since no patients showed thrombus in MRA. Zheng et al. [24] studied 56 patients with acute and subacute infractions and found that 39 of their patients had thrombus in M1 segment representing 55% of all patients; in addition, they found that M3 segment of middle cerebral artery was the least affected segment in MRA representing about 1.4%; they also found that M2 segment was the most affected segment developing thrombosis representing about 75% of patient followed by M3 segment. In contrast, the M1 was the least affected segment representing 25% keeping in mind that 67% of patients has multiple thrombi. Darwish et al. [25] studied 24 patients with MCA infraction in the 1st 24 h and found that M5 segment was the most affected segment and caudate nucleus was the least affected one. In the current study, SVS was not detected in 35 patients with ischemic stroke, this may be attributed to spontaneous recanalization of the thrombus, and in these patients, the thrombus size was to small and peripherally located and was beyond the level of detection. In a study done by Allibert R and his colleagues, they concluded that SWI is more sensitive than GRE in the visualization of occluded intracranial arteries during the acute phase of ischemic stroke [13]. In the current study, SWI was found to be more useful than MRA in defining the location of the thrombus and in determining its burden in the M1 segment, although a recent study has shown that the thrombus burden on SWI does not affect the success of endovascular recanalization therapy [28]. In our study we used SWI in the detection of multiple thrombi; compared with MRA, we found that 12 of 115 patients who had SVS in SWI had multiple thrombi; however, no one shows any distal thrombus in MRA. This may be attributed to decreased flow distally in the vessels due to proximal thrombus since the MRA depends on blood flow in the vessels, and also recanalization and small thrombus size may play a role in detecting these thrombi in MRA. Thus, SWI has high capability to detect the multiple thrombi compared to MRA with significant p value. In the current study and upon reviewing diffusion images, we found that 102 patients with DWI /SWI mismatch, and 68% of them developed infarction growth in follow up studies. In patients with no DWI/ SWI mismatch, no one of them developed infarction growth. All patients with positive mismatch had asymmetrical prominent vessel sign in SWI. These results are in agreement with El Nouby et al. [26] who studied 20 patients; 12 of them had asymmetrical prominent vessel sign matching DWI; on follow-up only 11 patients showed infarction growth with significant correlation the asymmetrical prominent vessel sign with infarction growth; these results also are in agreement with studies done by Chandrasekharan et al. [29] and Hung-Wen Kao et al. [30].
In a study done by Wang et al. [31], they found that a DWI-SWI mismatch has a similar ability as a DWI-PWI to detect the ischemic penumbra, which indicates that SWI can be used to forecast the ischemic penumbra of patients with acute ischemic cerebral stroke.
Limitations
Absent gold standard is a limitation for the current study; we depended on correlation with patient clinical data, diffusion and follow-up studies which confirmed the SWI findings.
Conclusions
SWI is more sensitive than TOF angiography for detecting peripheral thrombi in patients with acute ischemic stroke. Both techniques might complement each other for visual detection of the occluded vessel. We recommend implementation of SWI into routine acute stroke MRI protocols.
Availability of data and materials
The datasets used and or analyzed during the current study are available for the corresponding author on reasonable request.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- MRA:
-
Magnetic resonance angiography
- SWI:
-
Susceptibility weighted Imaging
- SVS:
-
Susceptibility vessel sign
- APVS:
-
Abnormally prominent vessel sign
- MCA:
-
Middle cerebral artery
- DWI:
-
Diffusion weighted imaging
- GRE:
-
Gradient echo
- TOF:
-
Time of flight
- DSA:
-
Digital subtraction angiography
- MIP:
-
Maximum intensity projection
References
Kim BJ, Kang HG, Kim H-J, Ahn S-H, Kim NY, Warach S, Kang D-W (2014) Magnetic resonance imaging in acute ischemic stroke treatment. J Stroke 16(3):131–145
Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, Alberts MJ, Zivin JA, Wechsler L, Busse O (1992) Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 32:78–86
Jörgensen L, Torvik A (1966) Ischaemic cerebrovascular diseases in an autopsy series. Prevalence, location and predisposing factors inverified thrombo-embolic occlusions, and their significance in the pathogenesis of cerebral infarction. J Neurol Sci 3:490–509
Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW (2005) Significance of susceptibility vessel sign on T2*weighted gradient echo imaging for identification of stroke subtypes. Stroke 36:2379–2383
Legrand L, Naggara O, Turc G, Mellerio C, Roca P, Calvet D, Labeyrie MA, Baron JC, Mas JL, Meder JF, Touzé E, Oppenheim C (2013) Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke 44:1878–1884
Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O (2011) The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 42:1775–1777
Krings T, Noelchen D, Mull M, Willmes K, Meister IG, Reinacher P, Toepper R, Thron AK (2006) The Hyperdense posterior cerebral artery sign: a computed tomography marker of acute ischemia in the posterior cerebral artery territory. Stroke 37:399–403
Ambrosius W, Gupta V, Kazmierski R, Hellmann A, Qian G, Nowinski WL (2011) The hyperdense posterior cerebral artery sign in CT is related to larger ischemic lesion volume. Pol J Radiol 76:13–17
Leary MC, Kidwell CS, Villablanca JP, Starkman S, Jahan R, Duckwiler GR, Gobin YP, Sykes S, Gough KJ, Ferguson K, Llanes JN, Masamed R, Tremwel M, Ovbiagele B, Vespa PM, Vinuela F, Saver JL (2003) Validation of computed tomographic middle cerebral artery “dot” sign: an angiographic correlation study. Stroke 34:2636–2640
Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, Shah SH, Towfighi A, Ovbiagele B, Kidwell CS, Tateshima S, Jahan R, Duckwiler GR, Viٌuela F, Salamon N, Villablanca JP, Vinters HV, Marder VJ, Saver JL (2011) CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 42:1237–1243
Radbruch A, Mucke J, Schweser F, Deistung A, Ringleb PA, Ziener CH, Roethke M, Schlemmer HP, Heiland S, Reichenbach JR, Bendszus M, Rohde S (2013) Comparison of susceptibility weighted imaging and TOF-angiography for the detection of thrombi in acute stroke. PLoS ONE 8:e63459
Gawad NK (2021) The added value of susceptibility weighted imaging in non traumatic pediatric brain lesions. Alexmed 3:1–2
Allibert R, Billon Grand C, Vuillier F, Cattin F, Muzard E, Biondi A, Moulin T, Medeiros E (2014) Advantages of susceptibility-weighted magnetic resonance sequences in the visualization of intravascular thrombi in acute ischemic stroke. Int J Stroke 9:980–984
Kang DW, Jeong HG, Kim DY, Yang W, Lee SH (2017) Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke 48:1554–1559
Flacke S, Urbach H, Keller E, Trنber F, Hartmann A, Textor J, Gieseke J, Block W, Folkers PJ, Schild HH (2000) Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology 215:476–482
Aoki J, Kimura K, Shibazaki K, Saji N, Uemura J, Sakamoto Y, Nagai K (2015) The susceptibility vessel sign at the proximal M1: a strong predictor for poor outcome after intravenous thrombolysis. J Neurol Sci 348:195–200
Aoki J, Kimura K, Shibazaki K, Sakamoto Y, Saji N, Uemura J (2013) Location of the susceptibility vessel sign on T2*-weighted MRI and early recanalization within 1 hour after tissue plasminogen activator administration. Cerebrovasc Dis Extra 3:111–120
Burden Park MG, Oh SJ, Baik SK, Jung DS, Park KP (2016) Susceptibility-weighted imaging for detection of thrombus in acute cardioembolic stroke. J Stroke 18:73–79
Gratz PP, Schroth G, Gralla J, Mattle HP, Fischer U, Jung S, Mordasini P, Hsieh K, Verma RK, Weisstanner C, El-Koussy M (2015) Whole-brain susceptibility-weighted thrombus imaging in stroke: fragmented thrombi predict worse outcome. AJNR Am J Neuroradiol 36:1277–1282
Payabvash S, Benson JC, Taleb S, Rykken JB, Hoffman B, McKinney AM, Oswood MC (2017) Susceptible vessel sign: identification of arterial occlusion and clinical implications in acute ischaemic stroke. Clin Radiol 72:116–122
Shinohara Y, Kinoshita T, Kinoshita F (2012) Changes in susceptibility signson serial T2*-weighted single-shot echo-planar gradient-echo images in acute embolic infarction: comparison with recanalization status on 3D time-of-flight magnetic resonance angiography. Neuroradiology 54:427–434
Abdelgawad MS, Aly RA (2020) Value of susceptibility-weighted MR imaging (SWI) in the detection of developmental venous anomaly. Egypt J Radiol Nucl Med 51, Article number 51:90
Hsu CC-T, Kwan GNC, Hapugoda S, Craigie M, Watkins TW, Haacke EM (2017) Susceptibility weighted imaging in acute cerebral ischemia: review of emerging technical concepts and clinical applications. Neuroradiol J 30(2):109–119
Zheng M-Z, Yang Q-Y, Lu X-D, Hu S-L, Chai C, Qin W (2019) Middle cerebral artery thrombus susceptibility-weighted imaging mapping predicts prognosis. Quant Imaging Med Surg 9:1556–1578
Darwish EA, Abdelhameed-El-Nouby M, Geneidy E (2020) Mapping the ischemic penumbra and predicting stroke progression in acute ischemic stroke: the overlooked role of susceptibility weighted imaging. Insights Imaging 11(1):1–12
Nouby MAE, Darwish EAF, Geneidi EAS (2018) The role of susceptibility weighted imaging (SWI) in evaluation of acute stroke. Egypt J Hosp Med 72(10):5398–5402
Park M-G, Oh S-J, Baik SK, Jung DS, Park K-P (2016) Susceptibility-weighted imaging for detection of thrombus in acute cardioembolic stroke. Journal of stroke 18(1):73
Weisstanner C, Gratz PP, Schroth G, Verma RK, Köchl A, Jung S (2014) Thrombus imaging in acute stroke: correlation of thrombus length on susceptibility-weighted imaging with endovascular reperfusion success. Eur Radiol 24:1735–1741
Kesavadas C, Santhosh K, Thomas B (2010) Susceptibility weighted imaging in cerebral hypoperfusion—can we predict increased oxygen extraction fraction? Neuroradiology 52:1047–1054
Kao H-W, Tsai FY, Hasso AN (2012) Predicting stroke evolution: comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol 22(7):1397–1403
Wang YR, Li ZS, Huang W, Yang HQ, Gao B, Chen YT (2021) The value of susceptibility-weighted imaging (SWI) in evaluating the ischemic penumbra of patients with acute cerebral ischemic stroke. Neuropsychiatr Dis Treat 17:1745–1750
Acknowledgements
We gratefully acknowledge the work efficiency and devotion of our imaging technicians which made this work possible.
Funding
No sources of funding.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the current study. EA and MAS contributed to study design, manuscript writing and image interpretation. EA and MAF performed manuscript revision, image interpretation and statistical analysis. AAA performed manuscript revision and image interpretation. MFA contributed to image revision, manuscript editing and image interpretation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the research ethics committee of Faculty of Medicine, at Minia University in Egypt on 2019 (reference number not applicable). All patients in this study gave a written consent to participate in this research project.
Consent for publication
Informed consent is given to publish data contained in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelgawad, E.A., Amin, M.F., Abdellatif, A. et al. Value of susceptibility weighted imaging (SWI) in assessment of intra-arterial thrombus in patients with acute ischemic stroke. Egypt J Radiol Nucl Med 52, 270 (2021). https://doi.org/10.1186/s43055-021-00649-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00649-0