Abstract
Background
Chronic subdural hematoma (CSDH) is a common situation in neurosurgical practice. Only a few studies had mentioned the opening of the inner membrane during the burr-hole evacuation of a CSDH. This study was designed to assess the benefits of inner membrane opening in the burr-hole evacuation of a CSDH and to find out if there is an added risk of such step that overweighs its benefits.
Methods
This is a descriptive cohort study that retrospectively reviewed 81 patients who underwent CSDH evacuation in Ain Shams University hospitals from October 2007 to August 2017.
Results
There were 54 (66.7%) males and 27 (33.3%) females. Age ranged from 40 to 84 years with a mean age of 64.95 years. The mean of maximum hematoma thickness measured in the preoperative brain CT scan was 22.58 mm ranging from 12 to 42 mm. Preoperative midline shift ranged from 0 to 21 mm with a mean value of 10.65 mm. All patients had an adequate radiological evacuation of the CSDH and did not develop a new acute subdural hematoma or intracerebral hematoma. The mean follow-up duration was 16.77 months (ranging from 3 to 60 months). There were 3 patients (3.7%) complicated with a recollection of subdural blood that required a second surgery. Included patients neither had postoperative cerebrospinal fluid leakage nor subdural empyema.
Conclusions
It can be concluded that this operative step was not a risk-adding but, apparently, a recurrence-preventing one. However, a prospective randomized controlled study is recommended to establish this finding.
Similar content being viewed by others
Background
Chronic subdural hematoma (CSDH) is a common situation in neurosurgical practice. The standard treatment for CSDH is its surgical evacuation, which usually results in neurological improvement. This condition has been treated by various surgical procedures such as burr-hole evacuation, twist-drill craniostomy, and craniotomy. However, all these procedures are associated with several complications including recurrence [1,2,3,4,5].
Burr-hole evacuation and closed-system drainage are the most common technique worldwide in evacuating a CSDH [1,2,3,4,5,6,7,8,9], but still, there is no consensus about many operative steps including the opening of the inner membrane of the subdural hematoma as there is no conclusive evidence of its benefits or risks.
Inner membrane opening was discussed during craniotomy for CSDH in many studies [10,11,12,13,14,15,16], but only a few studies had mentioned the opening of the inner membrane during the burr-hole evacuation of a CSDH [17, 18].
The aim of the study
The aim of the present study was to assess the benefits of inner membrane opening in the burr-hole evacuation of a CSDH and to find out if there is an added risk of such step that overweighs its benefits.
Methods
This is a descriptive cohort study that retrospectively reviewed the patients who underwent CSDH evacuation in Ain Shams University hospitals from October 2007 to August 2017. An approval from the research ethics committee of the Faculty of Medicine at Ain Shams University (reference number: FWA 00006444) was obtained. Furthermore, being a retrospective study, patients’ consents for participation and for publication were not applicable.
The study included adult patients (≥ 18 years) who underwent burr-hole evacuation of a unilateral CSDH and inner membrane opening.
Patients included in the study either had a normal coagulation profile or a defect that could be properly corrected prior to surgery. On the other hand, patients with bleeding tendencies that failed to be corrected prior to surgery were excluded from the current study.
The clinical data were collected from the patients’ charts and included the following: patients’ demographics, the apparent cause of the subdural hematoma, preoperative clinical state, laboratory findings, preoperative brain computed tomography (CT) scan findings, operative details, postoperative course and management of any complications, recollection of CSDH, follow-up CT scan findings, and the progressive notes in the follow-up visits.
CT scan appearance of the hematoma was classified into hypodense and isodense according to the brain parenchyma or a mixed density including hyperdense areas.
The preoperative and postoperative neurological status was classified according to the Markwalder’s Neurological Grading System [19].
In addition, assessment of the patients’ motor functions was documented according to the Medical Research Council (MRC) grading system [20]. However, for statistical purposes, only the most severely affected muscle group was considered as a representative of muscle power.
All the patients received prophylactic antiepileptic in the form of phenytoin (loading and maintenance doses) except in known hepatic patients, levetiracetam was used instead. Antiepileptic medications were continued for 6 months and then gradually withdrawn unless seizures occurred, in which case the antiepileptics were continued for 1 year after the last seizure.
Prophylactic intravenous antibiotics were administered 1 h prior to induction of anesthesia and continued for 72 h postoperatively followed by 5 days of oral antibiotic use.
Patients with preoperative thrombocytopenia < 100,000/μl received a platelet transfusion to increase the platelet count to ≥ 100,000/μl. Furthermore, antiplatelets were stopped on admission and restarted 4 weeks after surgery unless otherwise indicated. In such instance, patients received platelet transfusion intraoperatively even if their bleeding and clotting times were normal. Anemic patients received packed RBC transfusion to raise the hemoglobin to ≥ 10 g/dl.
Patients with preoperative prolonged prothrombin time (whether due to anticoagulant therapy or due to an intrinsic pathological coagulation defect) received fresh frozen plasma (FFP) to correct the international normalized ratio (INR) to ≤ 1.4.
All surgeries were done under general anesthesia with one or two coin-sized (about 2 cm in diameter) burr holes performed and located according to the operating surgeon’s decision based on the size and configuration of the hematoma.
The dura was coagulated by bipolar diathermy and then was incised in a cruciate fashion along with the outer membrane of the subdural hematoma, and then, the dural edges were coagulated, followed by gentle washing of the hematoma cavity by warm normal saline till the wash became clear.
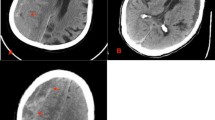
In at least in a single burr hole in each patient, the inner membrane was identified (Fig. 1), coagulated, and gently opened wide enough to allow evacuation and irrigation of the underlying contents which were lighter in color than the subdural fluid proper. In a few instances, an operating microscope was used for better visualization. The underlying arachnoid was left intact.
The subdural space was then filled with saline to wash out the subdural air. A suction drain catheter was placed over each burr hole letting the terminal end of the catheter in the subgaleal space. The catheter was pulled through a separate skin incision and sutured to the skin, then connected to its collecting chamber after compressing it to about 25% of its height to create a low negative pressure in the closed system for continuous drainage. No subdural catheter was used in any case. Wound closure was performed using full thickness interrupted sutures.
Postoperatively, patients remained flat on the bed for 2 days. The drain was removed 48–72 h after surgery.
After discharge from the hospital, follow-up visits were once every 2 weeks for 2 months and then once monthly afterward.
Follow-up brain CT was done on the 5th postoperative day, 2 weeks after discharge, and upon any reappearance of preoperative symptoms.
The recurrence rate was defined as the reappearance of symptomatic ipsilateral subdural reaccumulation with mass effect within 3 months after surgery.
Statistical analysis
Collected data were expressed as mean ± standard deviation and range and compared via a paired Student’s t test using SOFA statistics version 1.3.3 software.
Results
Out of the 81 patients enrolled in the current study, there were 54 (66.7%) males and 27 (33.3%) females. Age ranged from 40 to 84 years with a mean age of 64.95 years (standard deviation 10.16 years). A definite history of head trauma could be obtained from 46 (56.8%) patients while the rest of the patients failed to confirm such apparent etiology for the hematoma. Pertinent frequencies of different comorbidities and possible risk factors are illustrated in Table 1.
A left-sided hematoma was detected in 52 patients (64.2%) while 29 patients (35.8%) had right-sided hematomas. Sixty patients (74.1%) were treated with double burr holes while 21 patients (25.9%) were treated by a single burr hole. The mean of maximum hematoma thickness measured in the preoperative brain CT scan was 22.58 mm ranging from 12 to 42 mm. Preoperative midline shift ranged from 0 to 21 mm with a mean value of 10.65 mm. Table 2 illustrates the frequencies of different hematomas’ densities demonstrated on preoperative CT scans.
Regarding patients’ preoperative neurological status, 28 patients (34.6%) could be categorized as grade 1 according to the Markwalder’s Neurological Grading System while 36 patients (44.4%) were grade 2 and 17 patients (21.0%) were grade 3.
The brain CT scan on the 5th postoperative day for all patients confirmed an adequate evacuation of the CSDH and decrease in the midline shift and absence of any new acute subdural or intracerebral hematoma.
Each one of the included patients achieved a postoperative improvement to grade 0 denoting a statistically significant clinical improvement in response to surgical evacuation of the hematoma (p value < 0.001). The postoperative time lapsed to gain a grade 0 score according to the Markwalder’s Neurological Grading System ranged from 1 to 7 days with a mean of 2.49 days. Despite having normal speech documented for all patients by the end of the third postoperative month, the speech was affected (with variable degrees) preoperatively in 35 patients (43.2%). The pertinent mean values of motor power grades are illustrated in Table 3.
All patients had an adequate radiological evacuation of the CSDH and did not develop a new acute subdural hematoma or intracerebral hematoma. The mean follow-up duration was 16.77 months (ranging from 3 to 60 months). Postoperative seizures necessitating revision of antiepileptic medications were encountered in 10 patients (12.3%).
Out of the 81 patients included in the current study, there were 3 patients (3.7%) complicated with a recollection of subdural blood that required a second surgery. It is to be noted that the included patients neither had postoperative cerebrospinal fluid leakage nor were complicated with deep subdural infection (empyema). However, 3 patients (3.7%) had superficial wound infection that was effectively controlled with local wound care.
Discussion
The 81 patients included in this study underwent inner membrane opening during the burr-hole evacuation of their unilateral CSDH. There were no noted intraoperative or postoperative complications or risks related to the opening of the hematomas’ inner membrane.
All the patients in the present study achieved an improvement to grade 0 according to Markwalder’s Neurological Grading System within 1 week after surgery. All patients had an adequate evacuation of the CSDH and did not develop any new acute subdural or intracerebral hematoma. The recurrence rate of CSDH in this study was 3.7% which is a bit lower than the recurrence rate in the literature which ranges from 5 to 30% [3, 21].
Although CSDH is a common neurosurgical condition, still, there is no consensus on the best surgical steps among different neurosurgical centers and among neurosurgeons of the same center such as the number of burr holes or their size [22, 23], the use of irrigation [24], the site of the drain whether subdural or subgaleal, and the opening of the hematomas’ inner membrane.
The present study tried to assess the benefits and the risks of opening the hematomas’ inner membrane during the burr-hole evacuation of a CSDH. This operative step was barely mentioned in papers or textbooks. The low recurrence rate in the present study may be related to adequate evacuation of the subdural hematoma by opening the hematomas’ inner membrane till the arachnoid and the underlying brain became clear. The difference in color between the fluid over and under the inner membrane suggested that both cavities were not freely connected. This may signify that for adequate burr-hole evacuation of a CSDH, all membranes have to be opened till the arachnoid is seen.
Stanišic et al. [25] found that the recurrence rate in the first 3 months after CSDH evacuation through a single burr hole was 16% (17/107 patients), but they included bilateral cases and did not open the hematomas’ inner membrane.
Nnadi et al. [17] in their cross-sectional study on the incision of the inner membrane of the CSDH found no recurrence of the hematoma in any patient 3 months after surgery, but they included only 55 patients.
Tailor et al. [5] performed a retrospective study on burr-hole drainage of CSDH and found that the 6-month reoperation rate was 8.1% (10/123) in the subdural drain group. Incision of the hematomas’ inner membrane was not done, and 21.1% of cases were bilateral.
Kayaci et al. [18] in their retrospective study found that the recurrence rate was zero in the 144 patients who underwent incision of the inner membrane during the burr-hole evacuation of their CSDH, but they did not mention the follow-up period.
From the results of the current study and the two articles that discussed the opening of the inner membrane during a burr-hole evacuation of a CSDH [17, 18], it can be proposed that this operative step was not a risk-adding but, apparently, a recurrence-preventing one. A prospective randomized controlled study is recommended to establish this finding.
The limitations of this study were the retrospective nature and the absence of a control group.
Conclusions
This study proposed that inner membrane opening during the burr-hole evacuation of a CSDH was not a risk-adding but, apparently, a recurrence-preventing one.
Abbreviations
- CSDH:
-
Chronic subdural hematoma
- CT:
-
Computed tomography
- MRC:
-
Medical Research Council
References
Kale A, Öz İİ, Gün EG, Kalaycı M, Gül Ş. Is the recurrence rate of chronic subdural hematomas dependent on the duration of drainage? Neurol Res. 2017;39(5):399–402.
Xu C, Chen S, Yuan L, Jing Y. Burr-hole irrigation with closed-system drainage for the treatment of chronic subdural hematoma: a meta-analysis. Neurol Med Chir (Tokyo). 2016;56(2):62–8.
Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374(9695):1067–73.
Guilfoyle MR, Hutchinson PJ, Santarius T. Improved long-term survival with subdural drains following evacuation of chronic subdural haematoma. Acta Neurochir. 2017;159(5):903–5.
Tailor J, Fernando D, Sidhu Z, Foley R, Abeysinghe KD, Walsh DC. Clinical audit effectively bridges the evidence-practice gap in chronic subdural haematoma management. Acta Neurochir. 2017;159(4):627–31.
Liu W, Nicolaas A, Bakker MD, Groen MD. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014;121(3):665–73.
Yadav YR, Parihar V, Chourasia ID, Bajaj J, Namdev H. The role of subgaleal suction drain placement in chronic subdural hematoma evacuation. Asian J Neurosurg. 2016;11(3):214–8.
Chih AN, Hieng AW, Rahman NA, Abdullah JM. Subperiosteal drainage versus subdural drainage in the management of chronic subdural hematoma (a comparative study). Malays J Med Sci. 2017;24(1):21–30.
Oral S, Borklu RE, Kucuk A, Ulutabanca H, Selcuklu A. Comparison of subgaleal and subdural closed drainage system in the surgical treatment of chronic subdural hematoma. North Clin Istanb. 2015;2(2):115–21.
Sahyouni R, Mahboubi H, Tran P, Roufail JS, Chen JW. Membranectomy in chronic subdural hematoma: meta-analysis. World Neurosurg. 2017;104:418–29.
Balevi M. Organized chronic subdural hematomas treated by large craniotomy with extended membranectomy as the initial treatment. Asian J Neurosurg. 2017;12(4):598–604.
Shrestha P, Pant B, Shrestha P, Rajbhandari P. Organized subdural hematoma with thick membrane in chronic subdural hematoma. JNMA J Nepal Med Assoc. 2012;52(185):1–5.
Isobe N, Sato H, Murakami T, Kurokawa Y, Seyama G, Oki S. Six cases of organized chronic subdural hematoma. [Article in Japanese]. No Shinkei Geka. 2008;36(12):1115–20.
Rocchi G, Caroli E, Salvati M, Delfini R. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 2007;67(4):374–80.
Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011;50(2):103–8.
Mohamed EE. Chronic subdural hematoma treated by craniotomy, durectomy, outer membranectomy and subgaleal suction drainage. Personal experience in 39 patients. Br J Neurosurg. 2003;17(3):244–7.
Nnadi ON, Bankole OB, Olatosi JO. Chronic subdural hematoma: wide dural window and incision of inner membrane. Int J Med Res Health Sci. 2016;5:85–92.
Kayaci S, Kanat A, Koksal V, Ozdemir B. Effect of inner membrane tearing in the treatment of adult chronic subdural hematoma: a comparative study. Neurol Med Chir. 2014;54(5):363–73.
Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55(3):390–6.
Medical Research Council. Aids to the examination of the peripheral nervous system, Memorandum no. 45. London: Her Majesty's Stationery Office; 1981.
Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma. 2010;68(3):571–5.
Chandran RS, Nagar M, Sharmad MS, et al. Single parietal burr-hole craniostomy with irrigation and drainage for unilateral chronic subdural hematoma in young adults <40 years: a rationale behind the procedure. J Neurosci Rural Pract. 2017;8(3):389–94.
Han HJ, Park CW, Kim EY, Yoo CJ, Kim YB, Kim WK. One vs. two burr hole craniostomy in surgical treatment of chronic subdural hematoma. J Korean Neurosurg Soc. 2009;46(2):87–92.
Iftikhar M, Siddiqui UT, Rauf MY, Malik AO, Javed G. Comparison of irrigation versus no irrigation during burr hole evacuation of chronic subdural hematoma. J Neurol Surg A Cent Eur Neurosurg. 2016;77(5):416–21.
Stanišic M, Pripp AH. A reliable grading system for prediction of chronic subdural hematoma recurrence requiring reoperation after initial burr-hole surgery. Neurosurgery. 2017;81(5):752–60.
Funding
There was no funding or any financial support for the current study.
Availability of data and materials
The dataset supporting the conclusion of this article is included within the article (and in Additional file 1).
Author information
Authors and Affiliations
Contributions
The first author (AEDE) had a substantial role in the study design, data acquisition, and interpretation. In addition, the co-author (MAA) actively participated in the data acquisition, interpretation, and revision of the manuscript and was involved critically in the study design and drafting of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
An approval from the research ethics committee of the Faculty of Medicine at Ain Shams University (reference number: FWA 00006444) was obtained.
Consent for publication
Not applicable (the study does not involve identifiable human data).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
The excel worksheet containing data from the included patients. (XLSX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Elayouty, A.E.D., AbdelFatah, M.A. Inner membrane opening during the burr-hole evacuation of a chronic subdural hematoma: risk-adding or recurrence-preventing?. Egypt J Neurosurg 33, 4 (2018). https://doi.org/10.1186/s41984-018-0003-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-018-0003-x