Abstract
Tomato is one of the major cash crops in the Golapar area of district Nainital in Uttarakhand (India), where farmers are facing the problem of diseases in tomato cultivation. In the present investigation, a survey of tomato fields in the Golapar area of Haldwani block was conducted. The survey revealed the occurrence of late blight, early blight, stem rot, and wilt diseases causing an average loss of 80% to tomato. To counter the above diseases, Trichoderma harzianum (Th43), Pseudomonas fluorescens (Pf173), Jas mycorrhiza (AMF), and the fungicide (Mancozeb) in different combinations applyed through soil application (SA), seedling treatment (ST), and foliar spray (FA) were evaluated for growth promotion and disease control in tomato at experimental and farmers’ fields. The results of the study revealed that in experimental field, the maximum plant height (43.67 cm), highest number of branches (7.33) per plant, highest weight of fruit (47 g), highest number of fruits (39) per plant, minimum plant mortality (4% at 30 DAT and 3.2% at 30–60 DAT), minimum plant disease index (6.85), maximum total yield (256.00 q/ha), and marketable yield (246.67 q/ha) were observed in Th+Pf+JM (SA) + Th+Pf (ST) + Mancozeb (FS). At farmer’s field, minimum plant mortality (7.31%) at 30 days after transplanting (DAT) (5.73%) in 30–60 DAT, minimum plant disease index (11.47), and maximum yield 249.91 q/ha were observed in Th+Pf+JM (SA) + Th+Pf (ST) + Mancozeb (FS) combination. So, it can be concluded that among all the treatments, integrated treatment comprising of soil application of T. harzianum, P. fluorescens, Jas mycorrhiza (AMF) + seedling treatment with T. harzianum and P. fluorescens + three foliar sprays of Mancozeb was found very effective in reducing the plant mortality, promoting the plant growth, and increasing the yield at experimental field as well as at farmers’ fields.
Similar content being viewed by others
Background
Over 200 diseases have been reported to affect the tomato plants in the world (Watterson 1986). In Golapar area of district Nainital, early blight caused by Alternaria solani, late blight caused by Phytophthora infestans, wilts caused by Fusarium oxysporum and Ralstonia solanacearum, leaf curl, and mosaic are the most important diseases of tomato (Tewari et al. 2016). These diseases cause heavy losses in every crop season in the region. Chemicals have been used to manage the diseases in tomato over the years. Due to the excessive use of pesticides, the cost of crop production has also increased. Pesticides are toxic in nature and are equally harmful to human beings if applied injudiciously (Abhilash and Singh 2009). In the beginning, the use of chemical pesticides led to remarkable improvements in productivity. Later on, the non-target effect of these pesticides led to the development of resistance in pests, and residue in food chains and effect on human health and environment have been realized. Biological control may offer a good substitute to fungicides. This method involves the introduction of biocontrol agents (BCA) directly into the natural ecosystem or by adopting practices, which favor population build-up of naturally occurring BCAs. Combination of both approaches is probably ideal. In recent years, there has been tremendous progress in this area. Trichoderma is a major BCA and has been found effective to manage plant diseases (Harman 2000).
Fluorescent Pseudomonads are well-known PGPR and improve plant growth by a variety of mechanisms, including the production of siderophores, synthesis of antibiotics, production of plant growth hormones, enhancement of mineral uptake, and synthesis of enzymes that regulate plant ethylene levels (Glick 1995). Equipped with these abilities, fluorescent pseudomonads are also being exploited as potential crop protectants (Zegeye et al. 2011). Arbuscular mycorrhizal fungi (AMF) colonize roots of the majority of plant species and mutually benefit in a typical symbiotic relationship (Wang and Qiu 2006). They represent an interface between plants and soils, the mycelia of mycorrhiza grow both inside and outside the plant roots. AMF provide soil mineral nutrients (mainly phosphorus and nitrogen), water, and pathogen protection to the plant (Bonfante and Genre 2010).
In Golapar area of district Nainital, where tomato has since long been taken as a cash crop, the cost of cultivation has risen dramatically in view of the increased impact of diseases and pests (Fig. 1). Together with crop losses and increased cost of cultivation, farm gate price of the crop has increased resulting in diminishing margins. To counter the situation, farmers resort to indiscriminate use of pesticide but with a spiraling cost of production. However, of late, it is being widely perceived that no single technology, especially the use of synthetic chemicals, can lead to sustainable plant disease management rather the integration of multiple technologies can prove promising.
In Golapar area of district Nainital, Uttarakhand, where tomato has since long been taken as a cash crop, cost of cultivation has risen dramatically in view of the increased impact of above diseases and pests. Together with crop losses and increased cost of cultivation, farm gate price of the crop has increased resulting in diminishing margins. To counter the situation, farmers resort to indiscriminate use of pesticide but with a spiraling cost of production
Keeping the above into consideration, the present study was undertaken to develop eco-friendly management practices for the major diseases of tomato in Golapar area of district Nainital, India.
Materials and methods
To determine the disease incidence and severity in tomato, surveys were conducted during autumn and spring seasons 2013–2014, in a randomly selected 15 farmer’s field in 5 villages, namely, (1) Devalamalla, (2) Madanpur, (3) Sitapur, (4) Kunwarpur, and (5) Lachhampur of Golapar area of Haldwani block, district Nainital, Uttarakhand, India. This region has the highest area under tomato cultivation, and almost every farmer grows tomato as a cash crop.
Two experiments were conducted, the first was done at Vegetable Research Centre (VRC), G.B. Pant University of Agriculture and Technology, Pantnagar, Uttarakhand, India, during the autumn season of 2014–2015, whereas the second was conducted at five farmer’s fields in village Davalamalla, Golapar area of Haldwani block, district Nainital of Uttarakhand during autumn season of 2015–2016.Talc-based formulations of Trichoderma harzianum (Th 43), colony-forming units (CFU) count 2 × 107/g, and Pseudomonas fluorescens (Pf 173) CFU count 2 × 109/g were obtained from Biocontrol Laboratory, Department of Plant Pathology, G.B. Pant University of Agriculture and Technology, Pantnagar. Seeds of tomato (Solanum Lycopersicoum) cultivar TO-1458 (Syngenta India Ltd), the Jas mycorrhiza (Arbuscular mycorrhizal fungus) CFU count 100 propugales/g, M/S Shri Ram Solvent Extractions Pvt. Ltd., Uttarakhand) and fungicide INDOFIL M-45 (Mancozeb 75% WP) manufactured by INDOFIL, industries Ltd., Mumbai, used in both field experiments, were commercially procured. Experiments were set in a randomized block design (RBD), maintaining three replications for each treatment. The plot size was 2.5 × 2.5 m. A spacing of 50 cm between rows and plants and a total of 16 plants per plot were maintained. The texture of the soil of experimental field was sandy loam, and the pH was 6.6. Experimental field was prepared by one deep ploughing, followed by three harrowing and leveling leading to fine and well-pulverized soil. Before the final leveling of the field, NPK was applied in the form of urea (40 kg), single superphosphate (80 kg), and muriate of potash (60 kg) as a basal application. Urea as nitrogenous fertilizer was applied at 20 kg/ha each after 30 days of transplanting and 60 days of transplanting as a top dressing. Seeds of tomato cultivar TO-1458 were sown in a nursery bed for raising seedlings. Tomato seedlings were uprooted from the nursery beds 20 DAT. The seedlings were given root-dip treatment with spore/cell suspension prepared by mixing 10 g talc-based formulation of T. harzianum and P. fluorescens, respectively in 1 l of water for 30 min as per treatment. Vermicompost colonized with BCA was applied in the soil just before transplanting. Three foliar sprays of BCA/Mancozeb were given at 45, 60, and 75 days after transplanting (DAT). The untreated seedlings served as controls. To keep the experimental field free from insect pests (whitefly, aphids, and jassids), four sprays with 0.1% of methyldemeton (Metasystox) were applied at 25, 40, 60, and 80 DAT.
The following are the treatments at VRC:
-
1.
Th (SA + ST + FS)
-
2.
Pf (SA + ST + FS)
-
3.
Th (SA + ST) + Mancozeb (FS)
-
4.
Pf (SA + ST) + Mancozeb (FS)
-
5.
Th + Pf (SA + ST + FS)
-
6.
Th + JM (SA) + Th(ST + FS)
-
7.
Pf + JM (SA) + Pf (ST + FS)
-
8.
Th+ Pf +JM (SA) + Th + Pf (ST + FS)
-
9.
JM (SA) + Th + Pf (FS)
-
10.
JM (SA) + Mancozeb (FS)
-
11.
Th+ Pf (SA + ST) + Mancozeb (FS)
-
12.
Th + Pf +JM (SA) + Th+ Pf (ST) + Mancozeb (FS)
-
13.
Mancozeb (ST) + Mancozeb (FS)
-
14.
Control
(Th = Trichoderma harzianum (CFU count 2 × 107/g), Pf = Pseudomonas fluorescens (CFU count 2 × 109/g), JM = Jas mycorrhiza (CFU count 100 propugales/g), Mancozeb = INDOFIL M-45, SA = soil application, ST = seedling treatment, FS = foliar spray)
The following four effective treatments were further tested at farmers’ fields during the autumn season of 2015–2016 in the village Davlamalla of Golapar area in District Nainital
-
1.
Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS)
-
2.
Th + Pf + JM (SA) + Th + Pf (ST + FS)
-
3.
Th + Pf (SA + ST) + Mancozeb (FS)
-
4.
Th + Pf (SA + ST + FS)
-
5.
Control
Mode of application
Soil application (SA)
T. harzianum (CFU count 2 × 107/g) and P. fluorescens (CFU count 2 × 109/g) were multiplied on vermicompost (10/kg) separately as well as in combinations (5 g + 5 g/kg). The colonized vermicompost was applied (50 g/plant) in each plant just before transplanting. The Jas mycorrhiza (Glomus intraradices) (100 g/kg) mixed in vermicompost, with or without colonized with BCA, was applied50 g/plant in the soil just before transplanting (Singh and Zaidi 2002 and Kabdwal et al. 2017).
Seedling treatment (ST)
The seedlings were dipped in the suspension of Th (10 g/lit), Pf (10 g/lit), and Th + Pf (each with 5 g/lit) for 30 min just before transplanting in the field.
Foliar sprays (FS)
Th (10 g/lit), Pf (10 g/lit), Th + Pf (each with 5 g/lit), and Mancozeb (2.5 g/lit) were applied as foliar sprays. Battery-operated knapsack sprayer ASPEE VBD09 with a hollow cone nozzle was used for foliar spray. The constant pressure of 40 psi and uniform medium size droplet (225–325 μm) was maintained.
Observations
Data on (1) plant growth promotion, (2) disease incidence, (3) disease severity, and (4) total fruit yield was recorded. The observation on growth parameter was recorded at 45 DAT. Disease incidence was recorded on 30 DAT and 30–60 DAT. Disease severity was recorded at 70 DAT. The harvesting of tomato started on 65 DAT; after that, at an interval of 7 days, the total yield was estimated after final harvesting 120 DAT.
Disease incidence
Disease incidence was recorded for wilt and root rot diseases of tomato as under:
Disease severity
Disease severity for foliar blight of tomato crop was recorded based on the leaf parts affected at 0–5 scale: 0 = no visible lesion on leaf, 1 = up to 10%, 2 = 11–25%, 3 = 26–50%, 4 = 51–75%, and 5 = > 75% leaf area affected (Mayee and Datar 1986).
Five leaves from each plant and five plants/replicate plot were randomly selected to record disease severity, whereas the percent disease index (PDI) was calculated by using the following formula (Wheeler 1969):
\( \mathrm{Disease}\kern0.17em \mathrm{index}\;\left(\%\right)=\frac{\mathrm{Sum}\;\mathrm{of}\;\mathrm{all}\;\mathrm{numerical}\kern0.17em \mathrm{ratings}\;}{\mathrm{No}.\mathrm{of}\kern0.17em \mathrm{total}\kern0.17em \mathrm{leaves}\kern0.17em \mathrm{examined}\times \mathrm{maximum}\kern0.17em \mathrm{grade}}\times 100 \)
Yield
Ripened fruits were harvested at regular intervals from each plot, and total yield (q/ha) was estimated. Marketable yield was calculated by deducting damaged fruits from the total yield. Marketable yield (%) was calculated as:
Statistical analysis
The observations were recorded from three replications in a randomized block design. The data recorded in percentage were angularly transformed. Critical differences were calculated at 5% level of significance for comparison of treatment mean. All data were subject to analysis of variance (ANOVA) by using standard software STPR developed at Pantnagar University.
Results and discussion
In spring season, the highest incidence of disease (64%) was recorded in village Sitapur, while the lowest (57%) was in Lachhampur (Table 1). Maximum disease severity (29%) was recorded in village Madanpur, and the minimum (26%) was in Devalamalla. The mean disease incidence and severity considering all five villages were 60 and 27%, respectively. The incidence of diseases in tomato during autumn season varied from 66 to 95%, and the mean disease incidence and severity were recorded (85 and 62%, respectively) (Table 1). The lowest disease severity (40.87%) was found in village Davella malla, whereas the highest (80.74%) was in Lachampur. The data revealed that almost all the cultivated tomato variety in the five villages of Golapar area were diseased. Similar study was conducted by Ahmad et al. (2015) in Solan, Raipur, Bilaspur, Dur, Kanpur, Etah, and Barabanki where a survey was conducted to access the disease scenario in tomato crop during the years 2011 and 2012; it was reported that bacterial spot was predominant (26.4%), while Septoria leaf spot dominated (41.1%), early blight was recorded at a moderate level in both years (12.9 to 36.4%). Early blight was severe (80%) in Barabanki, followed by Etah and Kanpur, wherein the severity was 52.4 and 47.9%, respectively.
Effect of biocontrol agents and arbuscular mycorrhizal fungi on the plant growth of tomato
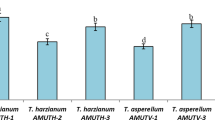
As shown in Table 2, the maximum plant height, number of branches/plant, fruit weight, and number of fruits/plant (43.67 cm,7.00 no, 47 g and 39 no) were recorded in the treatments Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS), followed by Th + Pf + JM (SA) + Th + Pf (ST + FS) (42.53 cm, 6.33 no, 43 g, and 38 no) and Th + Pf (SA + ST + FS) (41.27 cm, 6 no, 42 g, and 32 no) and were at par with each other but significantly different from control (29.47 cm, 4.00 no, 28 no, and 20 no), respectively. The present findings on increase in growth parameters of tomato are supported by Mwangi Margaret et al. (2011), Azarmi et al. (2011), and Singh et al. (2013) who reported an increase in plant vigor by the treatment of Th + AMF, Th, and Th + Ps, respectively.
Effect of BCAs, AMF, and fungicide alone or in combinations on tomato diseases under field conditions
To evaluate the relative effectiveness of disease control agents alone and in combination against soilborne (wilt, root rot complex) as well as foliar blight (early and late blight) of tomato, a field experiment was conducted during the autumn season of the year 2014–2015. Minimum plant mortality and maximum reduction on plant mortality at 30 DAT and 30–60 DAT were observed in the treatment Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS) (4, 80, and 3.87%), followed by Th + Pf + JM (SA) + Th + Pf (ST + FS) (5.75 and 6 and 73%), Th + Pf (SA + ST + FS) (5.74 and 7.71%), Mancozeb (ST) + Mancozeb (FS) (5.74 and 7.70%) and were at par with each other but significantly differed from other treatments and control (20 and 24%), respectively. Minimum plant disease index of foliar blight was observed in Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS) (6.85), followed by JM (SA) + Mancozeb (FS) (9.45), Th (SA + ST) + Mancozeb (FS) (9.49), Th + Pf (SA + ST) + Mancozeb (FS) (10.51), Mancozeb (ST) + Mancozeb (FS) (10.72), Pf (SA + ST) + Mancozeb FS (12.10), and Th + Pf + JM (SA) + Th + Pf (ST + FS) (12.29) and were at par with each other but significantly different from other treatments and control (43.82) Table 3.
Effect of BCAs, AMF, and fungicide alone and in combinations on the yield of tomato in field
Yield was also recorded as it is an important parameter of plant health. Data recorded on total yield and marketable yield at the experimental field are presented in Table 4 which reveal that all the treatments significantly superior with respect to total yield as well in marketable yield as compared to the control. Among the treatments, significant maximum (25,600 kg/ha) total yield and marketable yield (24,667 kg/ha) was observed in Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS), followed by Th + Pf + JM (SA) + Th + Pf (ST + FS) (27,233, 25,767 kg/ha), Th + Pf (SA + ST) + Mancozeb (FS) (26,767, 25,433 kg/ha), and Th + Pf (SA + ST + FS) (26,500, 25,200 kg/ha) and were at par with each other but significantly different from other treatments and control (19,500,15,200 kg/ha), respectively. Maximum percent share of marketable yield (96.36%) in total yield was observed in Mancozeb (ST) + Mancozeb (FS), followed by Th (SA + ST) + Mancozeb FS, Th + Pf (SA + ST + FS), Th + Pf (SA + ST) + Mancozeb (FS), AMF (SA) + Mancozeb (FS), and Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS).
Effect of selected treatments for the management of tomato diseases at farmers’ fields in Golapar area
During the year 2014–2015, the field evaluation of BCAs, AMF, and fungicide alone and in combination, the study revealed that Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS) was found the most effective, followed by Th + Pf + JM (SA) + Th + Pf (ST + FS), Th + Pf (SA + ST) + Mancozeb (FS), and Th + Pf (SA + ST + FS) in increasing plant growth, yield, and reduction of tomato diseases. These effective treatments (combinations) were further evaluated at Golapar area of district Nainital at five farmers’ field for confirmation of results during the autumn season 2015–2016. All treatments were found effective (Table 5) in reducing the plant mortality as well as foliar blight diseases in tomato. Minimum plant mortality and maximum reduction on plant mortality at 30 DAT, and 30–60 DAT were observed in Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS) (7.6 and 68.73%), followed by Th + Pf + JM (SA) + Th + Pf (ST + FS) (8% and 66.66%) and was at par with each other but significantly different from control (23 and 21%), respectively. Minimum plant disease index was observed in Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS) (11.47), followed by Th + Pf (SA + ST) + Mancozeb (FS) (11.80), Th + Pf + JM (SA) + Th + Pf (ST + FS + FS) (12.46), and Th + Pf (SA + ST) (11.80) and were at par with each other but significantly different from control (29.67) (Table 5). The yield data presented in Table 5 reveal that all the treatments significantly super in yield as compared to control. Among the treatments maximum (24,991 kg/ha) yield was observed in Th + Pf + JM (SA) + Th + Pf (ST) + Mancozeb (FS), followed by Th + Pf + JM (SA) + Th + Pf (SA + ST) (24,889 kg/ha) and Th + Pf (SA + ST + FS) (23,000 kg/ha) and were at par with each other but significantly different from control (15,353 kg/ha). These results are also in accordance with Srivastava et al. (2010) who reported that the combination of T. harzianum, P. fluorescens, and AMF gave significant disease reduction in incidence (70%), then control and increased in yield (20%) in tomato. Large numbers of chemicals have been used to manage the diseases over the years in tomato cultivation. In the beginning, these chemical pesticides led to remarkable improvements in productivity. Later on, the non-target effect of these pesticides led to the development of resistant in pests and residue in food chains and the effect on human health and environment have been realized (Abhilash and Singh 2009). Due to the excessive use of pesticides, the cost of crop production has also increased. The challenge today is how to achieve not only food security but also food safety by employing effective as well as environmentally benign measure for management of plant pathogens. Because of the abovementioned facts, an ecologically sound and cost-effective approach has been explored for sustainable plant disease management. The important component of this investigation was the use of the different combinations of locally available bioagents (BCAs): T. harzianum, P. fluorescens, and Mycorrhiza. Trichoderma species have long been recognized as biocontrol agents of plant diseases and for their multifarious capabilities viz. to increase the plant growth and development, high reproductive capacity, ability to survive under unfavorable conditions, high nutrient utilization efficiency, capacity to modify the rhizosphere, strong aggressiveness against plant pathogens, and efficacy in inducing plant defense mechanisms. Trichoderma species are producers of extracellular proteins and are best known for their ability to produce enzymes that degrade cellulose and chitin hence producing many useful byproducts (Harman and Kubicek 1998). Different strains of Trichoderma are known to produce more than 100 different metabolites with antibiotic potentials (Sivasithamparam and Ghisalberti 1998). Moreover, the ubiquitous presence of this genus at high population densities and its excellent rhizosphere competence, i.e., the ability to colonize and grow in association with plant roots leads to adaptability and its wider use in agriculture (Chet et al. 1997).
P. fluorescens is known to improve plant growth by a variety of mechanisms including production of siderophores, synthesis of antibiotics, production of plant growth hormones, enhancement of mineral uptake, and synthesis of enzymes that regulate plant ethylene levels (Kloepper et al. 1986). It is also being exploited as potential crop protectants. Borowicz et al. (1992) observed that the ability of plant growth-promoting fluorescent Pseudomonas inactivates cell wall degrading enzymes of plant pathogenic fungi. It is proved that antibiotic production is mainly responsible for the anti-fungal activity of P. fluorescens (Hebbar et al. 1992). The anti-fungal metabolite 2,4-diacetyl phloroglucinol produced by P. fluorescens plays a major role in the biological control of plant pathogens (Delany et al. 2000). Kell et al. (1992) indicated that the 2,4-diacetyl phloroglucinol produced by P. fluorescens suppresses soilborne plant pathogens in the rhizosphere.
Arbuscular mycorrhizal fungi (AMF or AM fungi) are found in the roots of about 80–90% of plant species and mutually benefit in a typical symbiotic relationship (Wang and Qiu 2006). AMF provide soil mineral nutrients (mainly phosphorus and nitrogen), water, and protection to the plant against the attack of plant pathogens (Bonfante and Genre 2010). Linderman (1994) reported that the prophylactic ability of AM fungi could be exploited in association with other rhizosphere microorganisms known to be antagonistic to root pathogens that are being used as biological control agents. The elicitation by an AM symbiosis of specific plant defense reactions could predispose the plant to an early response to attack by a root pathogen (Gianinazzi-Pearson et al. 1994). Among the compounds involved in plant defense (Bowles 1990) studied in relationship to AM formation are phytoalexins, enzymes of the phenylpropanoid pathway, chitinases, b-1,3-glucanases, peroxidases, pathogenesis-related (PR) proteins, callose, hydroxyproline-rich glycoproteins (HRGP), and phenolics (Gianinazzi-Pearson et al. 1994). Many studies suggested that microbial antagonists of fungal pathogens, either fungi or PGPR, do not antagonize AM fungi. Moreover, they can improve the development of the mycosymbiont and facilitate AM formation (Linderman 1994). This has been shown particularly for Trichoderma spp. (Calvet et al. 1993) and for Pseudomonas spp. producing 2,4-diacetylphloroglucinol (Vidal et al. 1996). Therefore, the combination of these three biocontrol agents improved plant growth and health (Barea and Jeffries 1995).
The study is demonstrating the method of using biocontrol agents (BCAs) along with safe chemical under the integrated disease management (IDM) practices for the management of major diseases of tomato. The result of the study was close agreement with the finding of Mwangi Margaret et al. (2011) who reported Th (P52) and AMF. In combination, significantly enhanced plant height, root dry weight, and reduced wilt pathogen caused by Fusarium oxysporum f.sp. lycopersici in tomato. The study was also supported with the study of Singh et al. (2013) who reported disease reduction (53.23%) and a significant increase in yield in tomato. They also observed that in the combination of T. harzianum and P. fluorescens enhanced the plant growth. Zghair et al. (2014) reported that seed treatment and foliar sprays of T. harzianum + P. fluorescens + Mancozeb gave lowest disease index. Thus, their combination not only suppressed the important pathogens of tomato but also promoted the growth of plant, which ultimately led to the increase in pesticide-free tomato production.
Conclusion
In the present study, soil application of T. harzianum (43), P. fluorescens (173), Jas mycorrhiza (AMF) + seedling treatment with T. harzianum (43), and P. fluorescens (173) + 3foliar spray of Mancozeb was found very effective in reducing the plant mortality, foliar blight, and high growth promotion activity increasing the yield of tomato at experimental field as well as farmers’ field. Therefore, the recommendation could be exploited under biointensive integrated disease management program for sustainable cultivation of tomato.
References
Abhilash PC, Singh N (2009) Pesticides use and application: an Indian scenario. J Hazard Mater 165:1–12
Ahmad M, Bhat M, Vennila S, Sardana HR, Khare CP (2015) Disease scenario of tomato in mid-hills and sub-tropical plains of India. Indian J Plant Protect 43(2):264–265
Azarmi R, Hajieghrari B, Giglou A (2011) Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr J Biotechnol 10:5850–5855
Barea JM, Jeffries P (1995) Arbuscular mycorrhizas in sustainable soil plant systems. In: Hock B, Varma A (eds) Mycorrhiza structure, function, molecular biology and biotechnology. Springer, Heidelberg, pp 521–559
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun 1:48. https://doi.org/10.1038/ncomms1046
Borowicz JJ, Pietr SJ, Stankiewcz M, Lewicka T, Zukowska Z (1992) Inhibition of fungal cellulase, pectinase and xylanase activity by plant growth-promotingfluorescentPseudomonas spp. Bulletin OILB SROP 15:103106
Bowles DJ (1990) Defense related proteins in higher plants. Annu Rev Biochem 59:873–907
Calvet C, Pera J, Barea JM (1993) Growth response of marigold (Tagetes erecta L.) to inoculation with Glomus mosseae, Trichoderma aureoviride and Pythium ultimum in a peat-perlite mixture. Plant Soil 148:1–6
Chet I, Inbar J, Hadar I (1997) Fungal antagonists and mycoparasites. In: Wicklow DT, Söderström B (eds) The Mycota IV: environmental and microbial relationships. Springer- Verlag, Berlin, pp 165–184
Delany I, Sheehan MM, Fenton A, Bardin S, Aarons SO, Gara F (2000) Regulation of production of the antifungal metabolite 2,4-diacetylphloroglucinol in Pseudomonas fluorescens F113: genetic analysis of ph1F as a transcriptional repressor. Microbiol Reading 146:537546
Gianinazzi-Pearson V, Gollotte A, Dumas-Gaudot E, Franken P, Gianinazzi S (1994) Gene expression and molecular modifications associated with plant responses to infection by arbuscular mycorrhizal fungi. In: Daniels M, Downic JA, Osbourn AE (eds) Advances in molecular genetics of plant-microbe interactions. Kluwer, Dordrecht, pp 179–186
Glick BR (1995) Enhancement of plant growth by free living bacteria. Can J Microbiol 41:109–117
Harman GE (2000) Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T- 22. Plant Dis 84:377–393
Harman GE, Bjoorjaman T (1998) Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enchancement in: Trichoderma and Gliocladium, vol, II, Harman, G. E. and Kubicek, C. K. 1998. (Eds.), Vol. 229, London: Taylor and Francis Ltd.
Hebbar KP, Davey AG, Dart PJ (1992) Rhizobacteria of maize antagonistic to Fusarium moniliforme, a soil-borne fungal pathogen; isolation and identification. Soil Biol Biochem 24:979987
Kabdwal BC, Tewari R, Sharma R, Kumar J (2017) A common minimum programme for biointensive pest/disease management in small farms of Uttarakhand (India). Asian J Agric Ext Econ Sociol 16(3):1–8
Kell C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Hass D, Defago G (1992) Suppression of root diseases by Pseudomonas fluorescens CHAO: importance of the bacterial secondary metabolite 2,4- diacetylphloroglucinol. Molec Plant Microbe Interact 5:413
Kloepper JW, Scher FM, Laliberti M, Tipping B (1986) Emergence promoting bacteria: description and implication for agriculture. In: Swinburne TR (ed) Iron Siderophore and plant disease. Planum, New York, pp 155–164
Linderman RG (1994) Role of VAM fungi in biocontrol. In: Pfleger FL, Linderman RG (eds) Mycorrhizae and plant health. APS, St Paul, pp 1–26
Mayee CD, Datar VV (1986) Phytopathometry technical bulletin-1. Marathwad Agril. Univ, Prabhani, p 25
Mwangi Margaret W, Monda Ethel O, Okoth SA, Jefwa Joyce M (2011) Inoculation of tomato seedlings with Trichodermaharzianum and arbuscular mycorrhizal fungi and their effect on growth and control of wilt in tomato seedlings. Braz J Microbiol 42:508–513
Singh SP, Singh HB, Singh DK (2013) Trichoderma harzianum and Pseudomonas sp. mediated management of Sclerotium rolfsii rot in tomato (Lycopersicon esculentum Mill.). Bio Scan 8(3):801–804
Singh US, Zaidi NW (2002) Current status of formulation and delivery of fungal and bacterial antagonists for disease management in India. In: Microbial biopesticide formulations and application. Project Directorate of Biological Control, Bangalore, p 269
Sivasithamparam K, Ghisalberti EL (1998) In: Kubicek CP, Harman GE (eds) Trichoderma and Gliocladium, Vol. 1, pp 139–191
Srivastava R, Khalid A, Singh US, Sharma AK (2010) Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f.sp. lycopersici for the management of tomato wilt. Biol Control 53:24–31
Tewari R, Kabdwal BC, Singh RP, Sharma R, Kumar J (2016) Current status of diseases and pesticide use in tomato: key source of income of farmers of Uttarakhand. Indian Phytopath 69(4s):680–682
Vidal MT, Andrade G, Azcón-Aguilar C, Barea JM (1996) Comparative effect of Pseudomonas, strain F113 [biocontrol agent antifungal] and its isogenic mutant, strain F113G22 [impaired biocontrol ability] on spore germination and mycelial growth of Glomus mosseae under monoxenic conditions. In: Azcón Aguilar C, Barea JM (eds) Mycorrhizas in integrated systems: from genes to plant development. European Commission, EUR 16728, Luxembourg, pp 673–676
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Watterson JC (1986) Diseases, the tomato crops. In: Atherton J, Rudich J (eds) . Champan and Hall Ltd, New York, pp 461–462
Wheeler BEJ (1969) An introduction to plant diseases. Wiley, London
Zegeye ED, Santhanam A, Gorfu D, Tessera M, Kassa B (2011) Biocontrol activity of Trichoderma viride and Pseudomonas fluorescens against Phytophthora infestans under greenhouse conditions. J Agric Technol 7(6):1589–1602
Zghair QN, Lal A, Nane MM, Sobita S (2014) Effect of bioagents and fungicide against early blight disease of tomato (Lycopersicon esculentum L.). international. Tunis J Plant Prot 7:330–333
Acknowledgements
Authors are thankful to Joint Director, VRC, Pantnagar, and farmers of Golapar area of District Nainital in Uttarakhand for providing land and other resources during the experiment and their kind cooperation, The valuable support from all the members of bio control lab of Plant Pathology department, GBPUAT Pantnagar is highly appreciated.
Funding
All India Coordinated Research Project on Biological Control funded by Indian Council of Agriculture Research.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Library of G.B. Pant University Pantnagar.
Author information
Authors and Affiliations
Contributions
All authors contributed equally in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kabdwal, B.C., Sharma, R., Tewari, R. et al. Field efficacy of different combinations of Trichoderma harzianum, Pseudomonas fluorescens, and arbuscular mycorrhiza fungus against the major diseases of tomato in Uttarakhand (India). Egypt J Biol Pest Control 29, 1 (2019). https://doi.org/10.1186/s41938-018-0103-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-018-0103-7