Abstract
Background
Patients with transient ischemic attack (TIA) or minor stroke are at risk of strokes. One of the precautionary measures to reduce risk of stroke in these patients is administration of antiplatelet drugs.
Methods
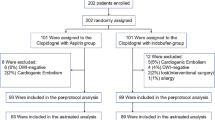
The aim of this study was comparison of clopidogrel with aspirin versus aspirin alone in prevention of stroke following TIA or acute minor stroke. This clinical trial study was carried out in Farshchian Hospital, Hamadan, Iran in 2018. Fifty-four patients with TIA, isolated visual attacks, and minor stroke were randomly assigned to receive aspirin 80 mg/day or aspirin 80 mg/day and clopidogrel 75 mg/day for 3 months.
Results
After this period, patients were examined for occurrence of myocardial stroke, hemorrhagic/ischemic stroke and drug complications. Ischemic stroke or TIA occurred in 5 (18.5%) patients in clopidogrel–aspirin group and 10 patients (37.1%) in aspirin group (P = 0.12).
Conclusion
The study suggests a trend toward superiority of administration of clopidogrel with aspirin versus aspirin alone in protection against secondary stroke. Studies in larger cohorts of patients are needed to verify these results.
Similar content being viewed by others
Background
Stroke is an incapacitating disorder commonly seen in the emergency departments all over the world [1]. Transient ischemic attacks (TIAs) are considered as important predictor of successive stroke and demise [2]. The increased risk of subsequent stroke in the three-month period after TIA has been estimated to be 18.5% which necessitates appropriate interventions [3]. Several studies have compared the effects of administration of different antiplatelet regimens in patients with former TIA. For more than two decades, aspirin has been acknowledged as an effective antiplatelet for decreasing the risk of secondary stroke [4]. Moreover, combination of clopidogrel and aspirin has been administered in different clinical settings for prevention of myocardial infarction, stroke, or mortality from cardiovascular events [5, 6]. The same dual regimen has been shown to be superior to administration of aspirin alone in prevention of stroke after TIA or acute minor stroke in a Chinese population [7]. However, the effect of such regimen in prevention of secondary stroke has not been assessed in Iranian population. A previous study in Iranian patients has assessed platelet aggregation and frequency of main adverse cardiovascular events 1 month after the angioplasty in patients treated with aspirin and clopidogrel. Authors reported complete response to clopidogrel in 65% of patients, intermediate resistance in 22% and complete resistance in the remaining. Notably, no main adverse cardiovascular event was detected [8]. Based on the substantial role of genetic and acquired factors in the determination of response to clopidogrel [9], population-based studies are needed to assess the effects of administration of clopidogrel with aspirin in reduction of risk of secondary stoke. Consequently, we conducted the current clinical trial to compare the effects of clopidogrel with aspirin versus aspirin alone in prevention of secondary stroke after TIA in Iranian patients.
Materials and methods
Patients
The current clinical trial was conducted in Neurology Department, Farshchian Hospital, Hamadan during 2018 (January–August). Patients who met the following criteria entered the study: (1) Diagnosis of thrombotic TIA (including isolated visual changes), (2) Scores less than 15 based on the National Institutes of Health Stroke Scale (NIHSS) [10], (3) ability to cooperate with the research team. Patients were excluded from the trial if they had any of the subsequent criteria: intracranial hemorrhage, main non ischemic brain disorder, contraindication to aspirin or clopidogrel, cardiac arrhythmia, valvular heart disease, any condition which necessitate carotid intervention. Consent form was filled out by all the individuals in this study and the study was confirmed by the local Ethical committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1397.301).
Study design
This was a randomized, double blind, parallel group study conducted in a University-affiliated hospital in Iran. A total of 54 patients diagnosed with TIA or acute minor stroke were randomized into two groups receiving either clopidogrel and aspirin or aspirin alone. Minor stroke was defined as the presence of NIHSS of 5 or less. No placebo was taken instead of clopidogrel in the aspirin alone group. The α and β values were set at 5% and 10% respectively.
The appropriate sample size was estimated from the following equation:
Z1−β = 1.28, Z1−α = 1.96, P1 = 10%, P0 = 18.5%
Risk of secondary stroke was considered as 18.5% for aspirin treated patients based on a previous study [3] and 10% for dual regimen [11].
Patients received either aspirin 80 mg/day or aspirin 80 mg/day and clopidogrel 75 mg/day for 3 months.
Outcomes measured
The odds and risk ratio of secondary TIA was measured in a 3-month period as the main outcomes. The adverse effects of each regimen were also recorded.
Statistical methods
Data were analyzed using SPSS v. 16 (IBM Corp, Chicago, IL, USA). P values less than 0.05 were considered as significant. Quantitative variables were described using mean and standard deviation (SD), whereas categorical variables were expressed as ratios or percentages. Chi square and student t tests were used for assessment of categorical and quantitative variables respectively.
Results
There were no substantial differences in the baseline features of the two groups, including type of TIA, TIA severity and sex ratio (Tables 1 and 2). No significant difference was detected in age of study participants (68.9 ± 10.5 in aspirin group, 65.7 ± 13.5 in aspirin + clopidogrel group, P = 0.321, df = 52, t = 1). Moreover, there were no significant differences in basic patient characteristics such as diabetes, hypertension, smoking, renal insufficiency between two groups of patients (P values > 0.05).
There was no significant difference in occurrence of secondary TIA in 3 month period between two groups [37.1% in aspirin group, 18.5% in aspirin + clopidogrel group, P = 0.129, df = 1, χ2 = 2.3, OR (95% CI) = 2.6 (0.8–9), RR (95% CI) = 1.7 (0.8–3.6)]. Odds ratio and risk ratio of secondary TIA in each group are shown in Table 3.
Two patients in aspirin group and no patient in the second group subsequently had myocardial infarction (P = 0.150, df = 1, χ2 = 2.07). Hemorrhagic stroke occurred in one patient in aspirin + clopidogrel group but no patient in the other group. There was no significant difference in adverse effects of drugs between two groups. All occurred adverse events are listed in Table 4.
Discussion
In spite of recent advances in diagnosis and treatment of cerebral ischemic events, these conditions are associated with both mortality and functional dependence [12]. Consequently, several studies have focused on identification of preventive modalities for their occurrence. Most of patients with ischemic stroke experience further episodes of stroke or TIA, instead of myocardial infarction. Consequently, antiplatelet therapies are suggested for prevention of secondary stroke [13]. The US Food and Drug Administration (FDA) have approved 4 drugs for protection against secondary stroke: monotherapy with aspirin, ticlopidine, clopidogrel, and dual administration of aspirin and extended-release dipyridamole [14]. Combinatory regimens with the aim of highest platelet inhibition in addition to vascular protection are proposed as the best strategy in this regard [13].
In the present study, we compared effects of clopidogrel with aspirin versus aspirin alone in the prevention of secondary stroke after TIA in a group of Iranian patients. Although patients received the combinatory regimen had lower occurrence of secondary stroke, the difference between two groups did not reach the significance level. Previous studies have reported superiority of combinatory regimen over aspirin alone in this regard in other populations [7]. Lack of significant difference between two groups in our current study might be explained by low sample size of the study. Alternatively, genetic- or ethnic-based differences in response to clopidogrel might be involved in the observed lower efficacy of this drug in Iranian patients.
Based on the acknowledged effects of CYP2C19 genetic polymorphism in determination of response to clopidogrel [15], FDA has recommended that clopidogrel must be administered with sufficient precautions in populations with a high prevalence of risk alleles [16]. Clopidogrel is activated in two phases and CYP2C19 has a critical role in this process. Genetic polymorphisms in the coding gene may result in the emergence of loss-of-function alleles, CYP2C19*2 and CYP2C19*3, and successively to the lack of the enzyme activity [17]. A previous study in Iranian population have shown relative high frequency of risk alleles in Iranian patients and stated that the FDA recommendations are more beneficial to be adapted in this country compared with other regions [18]. The frequencies of CYP2C19*2 and *3 were 23.4% and 3.9% in Iranian people, respectively [18]. However, the frequencies of CYP2C19 *2 and *3 have been 0.11–0.16 and 0.0–0.7 in Caucasian [19], 0.11% and 0.002% in Egyptians, 0.15% and 0.01% in Israeli Jews [20], and 0.13% and 0.03% in Lebanese [21], respectively. Another study has shown complete resistance in 13% of Iranian patients treated with clopidogrel which was not significantly different from other countries [8]. Future multi-center studies with larger sample sizes are necessary to explore the efficacy of mentioned combinatory regimen in prevention of secondary stroke in Iranian patients.
Conclusion
Based on our results, the frequency of hemorrhagic stroke and myocardial stroke as secondary events following TIA was not different between the study groups. Moreover, the combinatory regimen was not associated with higher rate of adverse effects. Consequently, our study demonstrates a trend toward superiority of administration of clopidogrel with aspirin versus aspirin alone in protection against secondary stroke after TIA in Iranian population and warrants future studies with larger sample sizes.
References
Johnston SC, Mendis S, Mathers CD (2009) Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 8(4):345–354
Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D et al (2005) Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 36(4):720–723
Coull AJ, Lovett JK, Rothwell PM, Oxford Vascular S (2004) Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 328(7435):326
Group IS (1997) The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet. 349(9065):1569–1581
Bhatt DL, Fox KAA, Hacke W, Berger PB, Black HR, Boden WE et al (2006) Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. New Engl J Med. 354(16):1706–1717
Investigators S (2012) Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. New Engl J Med. 367(9):817–825
Seadon S, Lang E (2015) Clopidogrel with aspirin versus aspirin alone in prevention of stroke following transient ischemic attack or acute minor stroke. Can J Emerg Med 17(3):315–317
Aghajani MH, Kobarfard F, Safi O, Sheibani K, Sistanizad M (2013) Resistance to Clopidogrel among Iranian patients undergoing angioplasty intervention. Iran J Pharm Res. 12:165–170
Ned RM (2010) Genetic testing for CYP450 polymorphisms to predict response to clopidogrel: current evidence and test availability: application: pharmacogenomics. PLoS Curr. 2:RRN1180
Goldstein LB, Bertels C, Davis JN (1989) Interrater reliability of the NIH stroke scale. Arch Neurol 46(6):660–662
Koziol K, Van der Merwe V, Yakiwchuk E, Kosar L (2016) Dual antiplatelet therapy for secondary stroke prevention. Use of clopidogrel and acetylsalicylic acid after noncardioembolic ischemic stroke. Can Fam Phys. 62(8):640–645
de Campos LM, Martins BM, Cabral NL, Franco SC, Pontes-Neto OM, Mazin SC et al (2017) How many patients become functionally dependent after a stroke? a 3-year population-based study in Joinville, Brazil. PLoS ONE 12(1):e0170204
Liao JK (2007) Secondary prevention of stroke and transient ischemic attack: is more platelet inhibition the answer? Circulation 115(12):1615–1621
Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K et al (2006) Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 37(2):577–617
Hulot J-S, Collet J-P, Silvain J, Pena A, Bellemain-Appaix A, Barthélémy O et al (2010) Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19* 2 loss-of-function allele or proton pump inhibitor coadministration: a systematic meta-analysis. J Am Coll Cardiol 56(2):134–143
Food Administration D. Drug safety communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm. 2011
Subraja K, Dkhar SA, Priyadharsini R, Ravindra BK, Shewade DG, Satheesh S et al (2013) Genetic polymorphisms of CYP2C19 influences the response to clopidogrel in ischemic heart disease patients in the South Indian Tamilian population. Eur J Clin Pharmacol. 69(3):415–422
Poopak B, Heidari M, Fallah P, Safari N, Rabieipoor S, Amiri Z et al (2016) Genetic polymorphisms of CYP2C19 and resistance to clopidogrel therapy among iranian patients suffering from ischemic heart disease. Res Mol Med. 4(3):41–46
Scott SA, Sangkuhl K, Shuldiner AR, Hulot JS, Thorn CF, Altman RB et al (2012) PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 22(2):159–165
Sameer AE, Amany GM, Abdela AA, Fadel SA (2009) CYP2C19 genotypes in a population of healthy volunteers and in children with hematological malignancies in Gaza Strip. Can J Clin Pharmacol. 16(1):e156–e162
Djaffar Jureidini I, Chamseddine N, Keleshian S, Naoufal R, Zahed L, Hakime N (2011) Prevalence of CYP2C19 polymorphisms in the Lebanese population. Mol Biol Rep. 38(8):5449–5452
Authors’ contributions
MT and SG-F wrote the manuscript. MK and MM supervised the study. FG analyzed the data. All authors read and approved the final manuscript.
Acknowledgements
The current study was supported by a Grant from Hamadan University of Medical Sciences.
Competing interests
The authors declare they have no competing interests.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Consent of publication
Not applicable.
Ethics approval and consent to participant
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Khazaei, M., Ghasemian, F., Mazdeh, M. et al. Comparison of administration of clopidogrel with aspirin versus aspirin alone in prevention of secondary stroke after transient ischemic attack. Clin Trans Med 8, 6 (2019). https://doi.org/10.1186/s40169-019-0223-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40169-019-0223-z