Abstract
Understanding the role of basal bodies (BBs) during development and disease has been largely overshadowed by research into the function of the cilium. Although these two organelles are closely associated, they have specific roles to complete for successful cellular development. Appropriate development and function of the BB are fundamental for cilia function. Indeed, there are a growing number of human genetic diseases affecting ciliary development, known collectively as the ciliopathies. Accumulating evidence suggests that BBs establish cell polarity, direct ciliogenesis, and provide docking sites for proteins required within the ciliary axoneme. Major contributions to our knowledge of BB structure and function have been provided by studies in flagellated or ciliated unicellular eukaryotic organisms, specifically Tetrahymena and Chlamydomonas. Reproducing these and other findings in vertebrates has required animal in vivo models. Zebrafish have fast become one of the primary organisms of choice for modeling vertebrate functional genetics. Rapid ex-utero development, proficient egg laying, ease of genetic manipulation, and affordability make zebrafish an attractive vertebrate research tool. Furthermore, zebrafish share over 80 % of disease causing genes with humans. In this article, we discuss the merits of using zebrafish to study BB functional genetics, review current knowledge of zebrafish BB ultrastructure and mechanisms of function, and consider the outlook for future zebrafish-based BB studies.
Similar content being viewed by others
Body of the primer
Zebrafish (Danio rerio): what is the basic phylogeny of this organism?
The zebrafish has been employed to study not only vertebrate development, genetics, and disease but, due to the comprehensive genomic annotation, has also helped answer questions of evolutionary diversity and phylogeny [1]. In short, zebrafish (Danio rerio), exhibit a toothless jaw that classifies them under the Cyprinidae family, with other members including carp, barbs, and minnows [2]. The Cyprinids themselves fall under the order of Cypriniformes, a large and diverse grouping of ray-finned (class: Actinopterygii ) bony freshwater fishes [3]. The presence of a swim bladder for buoyancy, moveable jaw, and symmetrical caudal fin classifies zebrafish under the subdivision (or infraclass) of Teleostei. There are currently approximately 26,840 species of Teleosts that represent 96 % of all living fish species spread across 40 orders, 448 families, and 4278 genera [4]. The successful evolutionary advance of Teleost fishes has been attributed to the occurrence of a whole genome duplication (WGD) that appeared early in the evolution of ray-finned fish, during the divergence from the lobe-finned fish, some 320–400 million years ago [5, 6]. It is generally accepted that WGD created new evolutionary opportunity by increasing gene number without affecting gene dosage [6]. Consequently, WGD allowed for the introduction of new loci with potentially advantageous functions, accounting for genetic redundancy. Whilst WGD created an expansion of genetic material and permitted leaps in evolutionary advancement, it has complicated analyses of gene function and phylogeny, especially in the context of human disease. Indeed, zebrafish possess at least one orthologue of approximately 70 % of all human genes (roughly 40 % of which have been duplicated) and 82 % of human disease causing genes [7]. However, idiosyncrasies taken into account, zebrafish offer a tractable system for studying gene function as indicated by the clear expansion in zebrafish functional genetics, notably in recent years, into the field of cilia and BB biology.
Basic basal body structure
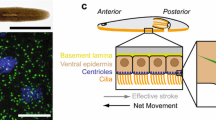
Consisting of a barrel-shaped centriole tethered to the cell membrane, the BB is fundamental in directing ciliogenesis, cell polarity, and providing a docking site for essential intraflagellar transport (IFT) proteins, required for appropriate ciliary function [8–10]. The centriole structure is highly conserved across species and is composed of nine triplet microtubules arranged in a cylindrical shape [11]. This structure forms the template that nucleates the ciliary axoneme. Therefore, correct BB construction dictates the development and function of the cilium. Much of the pioneering work on BB ultrastructure comes from detailed transmission electron microscopy (TEM) from the unicellular flagellate Chlamydomonas and the ciliated protozoa Tetrahymena [12, 13]. There is, however, very little high-resolution data on the ultrastructure of the BB in zebrafish and vertebrates as a whole. The majority of zebrafish TEM studies in the field of ciliogenesis have focused on axonemal structure of the cilium, which conforms to the nine plus two and nine plus zero doublets associated with motile and primary cilia, respectively [14]. Therefore, it might be speculated that BB structure also conforms to the nine triplet microtubular arrangement. Indeed, this is what is observed in BBs from modified primary cilia in the eye and motile cilia located in the choroid plexus, required for cerebrospinal fluid movement, in the brain (Fig. 1a–d) [15, 16]. Further conservation of structural function has been suggested from closer inspection of the cartwheel architecture, which forms the scaffold at the center of the BB. Sas-6, which localizes to the cartwheel that is required for early BB biogenesis in multiple model systems [17–20]. Interestingly, zebrafish Sas-6 protein has been observed to self-assemble in vitro into structures reminiscent of the cartwheel structure, suggesting Sas-6 itself is a major contributor to the core structural organization at the center of zebrafish BBs [21]. However, despite some compelling BB findings in zebrafish, further studies focusing on BB ultrastructure need to be conducted to elucidate BB structure variants between organisms and within different tissue types.
a–d Zebrafish transmission electron micrographs highlighting conserved BB structures: nine-triplet microtubule arrangement, TF transition fibers, DA distal appendages, DAV distal appendage vesicles. a Ultrastructure of the BBs and cilium from the zebrafish brain at 24 hpf. Scale bar 250 nm. b, c M-centrioles from zebrafish photoreceptors at 50 hpf. Scale bar 250 nm. d Schematic representation of zebrafish BB ultrastructure. e–g BBs and cilia can be simultaneously visualized in multiple zebrafish tissue types using GTU88 γ-Tubulin (BB) and acetylated α-Tubulin (cilia) antibodies. Fluorescent immunohistochemistry in the eye (e), pronephric duct (f), Kupffer’s vesicle (g) for BB (green), cilia (red) and nuclei (blue) in 24 hpf (e, f) 8 somite (g) embryos
Additional basal body structures or accessory structures
Electron microscopy has been fundamental to BB discovery. Descriptive TEM observations of Tetrahymena BBs nearly 50 years ago identified structural off-shoots that were speculated to be required for BB orientation and function [13]. These structures include the rootlet, basal foot, postciliary microtubules, transition fibers, and kinetodesmal fibers. Whilst the functional roles of these accessory structures remain largely unknown, there is growing evidence that they play a role in BB orientation, microtubular organization, ciliary structural support, and anchoring [22–24]. Some of these structures have been identified in zebrafish TEM, such as the rootlet, distal appendages, transition fibers, and basal foot (Fig. 1a–d) [16, 25]. However, the zebrafish model has yet to be exploited to specifically focus on accessory structure morphology and function.
Basal body origins and life cycle
BBs are closely related to centrosomes, they are structurally similar and both act as microtubule organizing centers. In fact, they are largely considered the same entity that has simply taken on a different cellular role post-mitotically, representing an efficient use of cellular components. It has yet to be determined when exactly in zebrafish development BBs become established. However, cilia are first observed during late epiboly, at the initiation of convergence and extension when cellular movements form the embryonic germ layers [26]. Despite this, it is well documented that the reassignment of occupation, from perinuclear centrosomal function to the apical membrane for ciliogenesis, occurs across species. Distinct cellular cues are likely to co-ordinate this event; however, the mechanism of centriole migration and BB docking to the apical membrane is not fully understood. Several studies in zebrafish have helped to identify some novel players in this process, including the Rac1 nucleotide exchange complex ELMO–DOCK1, and the Hippo pathway [27, 28]. Functional knockdown of elmo1, dock1, or ezrin1 (components of the ELMO–DOCK1 complex), using antisense morpholino oligonucleotides, results in morphological defects consistent with cilia loss [27]. Morphant embryos display detached BBs at the apical membrane and impaired ciliary axoneme formation. Similarly, the Hippo pathway transcriptional co-activator yes-associated protein (yap) has been shown to be required for appropriate BB arrangement and apical membrane docking during zebrafish ciliogenesis [29]. Examination of the cross-talk and interactions between the proteins proposed to orchestrate correct BB migration and docking will help clarify this poorly understood process.
Duplication of BBs occurs during mitosis. In multiciliated cells (MCCs), BB number directly underpins the sum of motile cilia, thus proposing the quandary; how do multiple BBs form without cell division? Deuterosomes, electron-dense structures, are believed to drive centriole amplification in MCCs [30]. Deuterosomes have yet to be directly observed in zebrafish and it may be speculated that an alternative method for centriole amplification is employed here. Indeed, Deuterosome protein 1 (Deup1), required for deuterosome-dependent (DD) centriole biogenesis, is not present in zebrafish [31]. Interestingly, cep63 required for mother centriole duplication (MCD) is present in zebrafish [31]. Deup1 and cep63 are known to be paralogues with divergent roles in MCC promotion. The presence of cep63 but not deup1 in zebrafish suggests that Deup1 arose from cep63 and that zebrafish amplify their centrioles via MCD, this is likely since zebrafish MCC only contain a few cilia [31]. However, what environmental cues instruct a cell to start amplifying centrioles? Cells are singled out to become MCC through inhibition of notch/delta signaling. Notch regulates Multicilin that promotes the production of centriolar structural proteins and foxj1, required for basal body docking, cilia formation and motility [32, 33]. In zebrafish, the foxj1a (the homologue of the mammalian Foxj1) target geminin coiled-coil domain containing (gmnc) has been identified to be required for MCC formation [32]. Fish with disrupted gmnc fail to generate MCC, lack cells containing multiple BBs and develop cystic kidneys, due to the requirement for MCCs to propel filtrate along the zebrafish pronephric tubule [32]. This suggests that gmnc is a critical regulator of centriole amplification. Thus, a cascade of gene regulation is required to promote centriole amplification and ultimately MCC commitment. However, the regulated decision to activate this cascade, independent of cell division, remains unclear.
Identification of basal body components
Determining the structural protein composition of BBs has often been a complex task, mainly due to difficulties isolating matrix-embedded centrioles from surrounding contaminants for proteomic analysis. In particular, proteins that make up the amorphic pericentriolar material (PCM) can often obscure centriole-specific proteins [34], However, some clever approaches have been used to piece together the ingredients that make up the BB. Several studies have taken a comparative genomics approach to identify genome differences between ciliated and non-ciliated species [35, 36]. Whilst this predicts the required ciliary components, it does not dissect out BB-specific centriolar proteins. A much more direct approach has been used in Tetrahymena and Chlamydomonas, where minimal PCM has aided BB isolation allowing mass spectroscopy to identify more specific BB proteome candidates [34, 37]. This has been highly informative in identifying a “parts list” for basal body assembly. Whilst similar experiments have not been conducted in zebrafish, high conservation in centriole function and therefore protein content should permit vertebrate follow-up experiments. In recent years, the multinational consortium known as SYSCILIA has compiled a “Gold standard” (SCGS) list of ciliary components found in the human genome [38]. For this article and to aid researchers wishing to study BB function in zebrafish, we have extracted BB- and centrosome-specific genes from the SCGS list and cross-referenced against genes with functional data in zebrafish (Table 1). Out of the 60 BB-/centrosome-specific proteins extracted from the SCGS list, 29 showed zebrafish functional follow-up studies, with the majority limited to knockdown as opposed to knockout approaches of gene silencing. It is clear from our table that BB researchers are just beginning to realize the power of zebrafish to study vertebrate function of BB genes. With advanced genome editing techniques now accessible in zebrafish, we expect some insightful BB zebrafish papers to follow.
Notable basal body findings
Forward genetic mutagenic screens performed in the 1990’s, spearheaded zebrafish to the forefront of vertebrate functional genetic research. Teams from Boston (USA) and Tubingen (Germany), lead by Wolfgang Driever and Christiane Nusslein-Volhard, recovered hundreds of N-ethyl-N-nitrosourea (ENU) directed mutations that caused gross morphological abnormalities in zebrafish development [39, 40]. At the time of screening, the significance of cilia in human disease had not been determined. Mutants identified through screening processes were grouped together based on common phenotypic features. One group of mutants showed phenotypic similarities to the ift88 mouse, a gene that had been shown in chlamydomonas to be required for ciliogenesis. Now considered the archetypal zebrafish ciliopathy phenotype, mutant lines display randomized heart looping and laterality defects, curved body axis, hydrocephalus, pronephric and glomerular cysts, and defective eye development [41]. Several of these mutations have been mapped to key components in ciliary processes. Notably affecting components of the IFT system. For example, the zebrafish mutants oval (ift88), fleer (ift70), and elipsa (traf3ip1), display loss of ciliary assembly [42–45]. However, these mutants have intact BBs, suggesting that the BB alone is not sufficient for ciliogenesis.
Early zebrafish ENU screens appeared to recover mainly ciliary/axonemal gene mutations, rather than those specific to basal body construction or function, although a number of mutants still remain unmapped. More BB/centriolar relevant mutants have been discovered through genetic screens for maternal-effect mutations [46, 47]. These experiments set out to understand the maternal factors required for early embryonic development and in doing so, identify genes involved in the early cell cycle events that occur before zygotic genes switch on. As previously mentioned, cilia do not form in zebrafish until late gastrulation (approximately 9-h post-fertilization (hpf)), suggesting that any centriolar mutations will be more akin to the centrosome [26]. Interestingly, one of the mutants recovered, a missense mutant (Asn414Lys) known as cellular atoll (cea), encodes the centriolar component Sass6 [48]. Genotypically homozygote cea individuals develop to adults and look phenotypically identical to wildtype, however females produce clutches of eggs that due to defects in centrosomal duplication arrest during early cell division. Thus, Sass6 is a maternal effect gene required for pre-gastrulation centrosomal duplication in zebrafish. However, the single amino acid change in cea appears not to affect BB function, homozygotes are viable and develop to adulthood. In other organisms, Sas-6 is localized to the centriolar cartwheel and has been speculated to form the cartwheel hub where loss leads to aberrant triplet microtubule numbers [19, 20, 49]. Thus, Sas-6 localizes to the cartwheel hub and is essential for centriole symmetry. Indeed, x-ray crystallography of zebrafish Sas-6 N-terminal has revealed that it assembles itself in vitro into constructs reminiscent of cartwheel hubs [21]. Further work on zebrafish, with the development of conditional mutations, will be critical in understanding the role of vertebrate Sas-6 in BB function.
Zebrafish forward genetic screens have been instrumental in understanding gene function, however mutations for genes of interest are not always recovered. A popular choice, although recently called under scrutiny, is the use of antisense morpholino oligonucleotide technology (MO) to block gene-specific translation [50, 51]. MOs are cheap to synthesize, easy to administer and fast to generate preliminary data. Furthermore, since MOs provide gene knockdown rather than knockout their use maybe more favorable for understanding gene function required for very early stages of development, such as cellular division, when early lethality otherwise masks ENU mutation recovery. Several zebrafish studies have utilized MOs to study basal body protein function in vertebrates. A notable case is that of Poc1, a core centriolar WD40 domain protein identified in both Chlamydomonas and Tetrahymena centriolar proteomic screens [34, 37, 52]. Interrogation of Poc1b function in Tetrahymena revealed a structural role in BB stability [53]. Knockdown of the zebrafish orthologue Poc1b using MOs results in phenotypic similarities to cilia deficient mutants, including visual impairment. Cilia motility and length is hindered in Poc1b morphant zebrafish embryos [53–55]. Recently, mutations in POC1B have been identified in patients displaying ciliopathy features [54, 56, 57]. Together, these data show the power of multidisciplinary research that can ultimately lead to the identification of novel disease causing genes.
Strengths and future of basal body research in zebrafish
The many advantages of using zebrafish as a model organism has firmly established this small tropical aquarium fish as a popular laboratory aid. Their rapid development, production of large numbers of eggs, optical transparency and excellent value for money are very appealing to vertebrate researchers. Additionally, BBs can be easily visualized alongside cilia in multiple zebrafish tissue by using primary antibodies for γ-Tubulin (BB—GTU88 Sigma) and acetylated α-Tubulin (Cilia—T6793 Sigma) in conjunction with isotype-specific secondary antibodies (Fig. 1e–g) [58]. For many years, a major drawback when modeling gene function in zebrafish was the difficulty in performing targeted mutagenesis. As such, zebrafish researchers have relied on MOs to knockdown gene-specific translation, a relatively quick and inexpensive technique [59]. However, problems associated with MO off-target defects have meant that an arduous list of controls need implementing in order to validate MO induced phenotypic changes [60, 61]. In the last few years, techniques to provide targeted mutagenesis in zebrafish have rapidly evolved thanks to the use of genome editing tools such as TALENS and CRISPR [62, 63]. Their development has highlighted some of the inaccuracies in the literature that have spread through MO use, where as many as 80 % of MOs may actually fail to recapitulate bona fide mutations in genes of interest [50]. CRISPR and TALENS take advantage of the imperfect endogenous repair mechanism, non-homologous end joining, which initiates after targeted double stranded DNA breaks are induced by certain endonucleases (reviewed in: [64, 65]). The development of tissue-specific promoter driven endonuclease expression has enabled researchers to create conditional mutants [66]. Minimal knowledge of molecular biology is required to generate the reagents required to direct the CRISPR Cas9 endonuclease to a favorable region of the genome, making this available to most laboratories and favorable over TALENS. In addition, there are comprehensive published protocols to perform, validate, and maintain CRISPR-induced mutagenic lines [66, 67]. Therefore, generating CRISPR directed mutant zebrafish lines is fast becoming an established method in zebrafish laboratories. Yet, there is little published work on BB-specific mutant zebrafish lines. Both global and conditional CRISPR techniques will provide BB researchers with invaluable tools to study candidate gene function, especially when considering the ubiquitous nature of BB gene expression. There is huge scope for utilizing zebrafish in BB research and it will be exciting to see how the systematic mutagenesis of the BB proteome will identify novel roles both at the structural and functional level.
Ethics statement
Animal maintenance, husbandry, and procedures are defined and controlled by the Animals (Scientific Procedures) Act 1986. All animal experimentation has been carried out under licenses granted by the Home Secretary (PPL No. 70/7892) in compliance with Biological Services Management Group and the Biological Services Ethical Committee, SGUL, London, UK.
Abbreviations
- BB:
-
basal bodies
- WGD:
-
whole genome duplication
- IFT:
-
intraflagellar transport
- TEM:
-
transmission electron microscopy
- MCC:
-
multiciliated cells
- PCM:
-
pericentriolar material
- SGSC:
-
Syscilia’s Gold Standard
- ENU:
-
N-ethyl-N-nitrosourea
- Hpf:
-
hours post-fertilization
- MO:
-
antisense morpholino oligonucleotide
- TALENs:
-
transcription activator-like effector nucleases
- CRISPR:
-
clustered, regularly interspaced, short palindromic repeat
- JSRD:
-
joubert syndrome and related disorders
- BBS:
-
bardet biedl syndrome
- T2D:
-
type 2 diabetes
- ADPKD:
-
autosomal dominant polycystic kidney disease
- NPHP:
-
nephronophthisis
- AS:
-
Alström Syndrome
- OFDS:
-
Orofaciodigital syndrome type 1
- MKS:
-
Meckels syndrome
- RP:
-
Retinitis pigmentosa
- LCA:
-
Leber’s congenital amaurosis
- MC:
-
microcephaly
- USH2A:
-
Usher syndrome 2a
- COACH:
-
cerebellar vermis oligophrenia ataxia coloboma hepatic fibrosis
- SCZD:
-
schizophrenia
- SLS:
-
Senior-Loken syndrome
- O:
-
osteopetrosis
- CORD:
-
cone-rod dystrophy
- RD:
-
retinal degeneration
References
McCluskey BM, Postlethwait JH. Phylogeny of zebrafish, a “model species,” within Danio, a “model genus”. Mol Biol Evol. 2015;32(3):635–52.
Winfield IJ, Nelson JS. Cyprinid fishes: systematics, biology and exploitation. London: Chapman and Hall; 1991.
Near TJ, Eytan RI, Dornburg A, Kuhn KL, Moore JA, Davis MP, Wainwright PC, Friedman M, Smith WL. Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci USA. 2012;109(34):13698–703.
Nelson JS. Fishes of the world. 4th ed. Hoboken: Wiley; 2006.
Ravi V, Venkatesh B. Rapidly evolving fish genomes and teleost diversity. Curr Opin Genet Dev. 2008;18(6):544–50.
Glasauer SM, Neuhauss SC. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol Genet Genomics. 2014;289(6):1045–60.
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503.
Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40(1):69–77.
Li S, Fernandez JJ, Marshall WF, Agard DA. Three-dimensional structure of basal body triplet revealed by electron cryo-tomography. EMBO J. 2012;31(3):552–62.
Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol. 2001;11(20):1586–90.
Hodges ME, Scheumann N, Wickstead B, Langdale JA, Gull K. Reconstructing the evolutionary history of the centriole from protein components. J Cell Sci. 2010;123(Pt 9):1407–13.
Geimer S, Melkonian M. The ultrastructure of the Chlamydomonas reinhardtii basal apparatus: identification of an early marker of radial asymmetry inherent in the basal body. J Cell Sci. 2004;117(Pt 13):2663–74.
Allen RD. The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J Cell Biol. 1969;40(3):716–33.
Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development. 2005;132(8):1907–21.
Wilkinson CJ, Carl M, Harris WA. Cep70 and Cep131 contribute to ciliogenesis in zebrafish embryos. BMC Cell Biol. 2009;10:17.
Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. 2015;17(3):228–40.
Culver BP, Meehl JB, Giddings TH Jr, Winey M. The two SAS-6 homologs in Tetrahymena thermophila have distinct functions in basal body assembly. Mol Biol Cell. 2009;20(6):1865–77.
Leidel S, Delattre M, Cerutti L, Baumer K, Gonczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat Cell Biol. 2005;7(2):115–25.
Nakazawa Y, Hiraki M, Kamiya R, Hirono M. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr Biol. 2007;17(24):2169–74.
Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, Glover DM. DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr Biol. 2007;17(17):1465–72.
van Breugel M, Hirono M, Andreeva A, Yanagisawa HA, Yamaguchi S, Nakazawa Y, Morgner N, Petrovich M, Ebong IO, Robinson CV, et al. Structures of SAS-6 suggest its organization in centrioles. Science. 2011;331(6021):1196–9.
Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol. 2005;25(10):4129–37.
Clare DK, Magescas J, Piolot T, Dumoux M, Vesque C, Pichard E, Dang T, Duvauchelle B, Poirier F, Delacour D. Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat Commun. 2014;5:4888.
Stinchcombe JC, Randzavola LO, Angus KL, Mantell JM, Verkade P, Griffiths GM. Mother centriole distal appendages mediate centrosome docking at the immunological synapse and reveal mechanistic parallels with ciliogenesis. Curr Biol: CB; 2015.
Hansen A, Zeiske E. The peripheral olfactory organ of the zebrafish, Danio rerio: an ultrastructural study. Chem Senses. 1998;23(1):39–48.
Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12(4):407–12.
Epting D, Slanchev K, Boehlke C, Hoff S, Loges NT, Yasunaga T, Indorf L, Nestel S, Lienkamp SS, Omran H, et al. The Rac1 regulator ELMO controls basal body migration and docking in multiciliated cells through interaction with Ezrin. Development. 2015;142(1):174–84.
Toret CP, Collins C, Nelson WJ. An Elmo-Dock complex locally controls Rho GTPases and actin remodeling during cadherin-mediated adhesion. J Cell Biol. 2014;207(5):577–87.
He L, Xu W, Jing Y, Wu M, Song S, Cao Y, Mei C. Yes-associated protein (Yap) is necessary for ciliogenesis and morphogenesis during pronephros development in zebrafish (Danio Rerio). Int J Biol Sci. 2015;11(8):935–47.
Dehring DA, Vladar EK, Werner ME, Mitchell JW, Hwang P, Mitchell BJ. Deuterosome-mediated centriole biogenesis. Dev Cell. 2013;27(1):103–12.
Zhao H, Zhu L, Zhu Y, Cao J, Li S, Huang Q, Xu T, Huang X, Yan X, Zhu X. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat Cell Biol. 2013;15(12):1434–44.
Zhou F, Narasimhan V, Shboul M, Chong YL, Reversade B, Roy S. Gmnc is a Master Regulator of the Multiciliated Cell Differentiation Program. Curr Biol. 2015;25(24):3267–73.
Ma L, Quigley I, Omran H, Kintner C. Multicilin drives centriole biogenesis via E2f proteins. Genes Dev. 2014;28(13):1461–71.
Keller LC, Romijn EP, Zamora I, Yates JR 3rd, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Curr Biol. 2005;15(12):1090–8.
Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117(4):527–39.
Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117(4):541–52.
Kilburn CL, Pearson CG, Romijn EP, Meehl JB, Giddings TH Jr, Culver BP, Yates JR 3rd, Winey M. New Tetrahymena basal body protein components identify basal body domain structure. J Cell Biol. 2007;178(6):905–12.
van Dam TJ, Wheway G, Slaats GG, Huynen MA, Giles RH. The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia. 2013;2(1):7.
Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46.
Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36.
Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125(23):4655–67.
Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10(4):437–44.
Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18(11):4353–64.
Tsujikawa M, Malicki J. Genetics of photoreceptor development and function in zebrafish. Int J Dev Biol. 2004;48(8–9):925–34.
Borovina A, Ciruna B. IFT88 plays a cilia- and PCP-independent role in controlling oriented cell divisions during vertebrate embryonic development. Cell Rep. 2013;5(1):37–43.
Dekens MP, Pelegri FJ, Maischein HM, Nusslein-Volhard C. The maternal-effect gene futile cycle is essential for pronuclear congression and mitotic spindle assembly in the zebrafish zygote. Development. 2003;130(17):3907–16.
Dosch R, Wagner DS, Mintzer KA, Runke G, Wiemelt AP, Mullins MC. Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell. 2004;6(6):771–80.
Yabe T, Ge X, Pelegri F. The zebrafish maternal-effect gene cellular atoll encodes the centriolar component sas-6 and defects in its paternal function promote whole genome duplication. Dev Biol. 2007;312(1):44–60.
Strnad P, Gonczy P. Mechanisms of procentriole formation. Trends Cell Biol. 2008;18(8):389–96.
Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 2015;32(1):97–108.
Stainier DY, Kontarakis Z, Rossi A. Making sense of anti-sense data. Dev Cell. 2015;32(1):7–8.
Keller LC, Geimer S, Romijn E, Yates J 3rd, Zamora I, Marshall WF. Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol Biol Cell. 2009;20(4):1150–66.
Pearson CG, Osborn DP, Giddings TH Jr, Beales PL, Winey M. Basal body stability and ciliogenesis requires the conserved component Poc1. J Cell Biol. 2009;187(6):905–20.
Beck BB, Phillips JB, Bartram MP, Wegner J, Thoenes M, Pannes A, Sampson J, Heller R, Gobel H, Koerber F, et al. Mutation of POC1B in a severe syndromic retinal ciliopathy. Hum Mutat. 2014;35(10):1153–62.
Zhang C, Zhang Q, Wang F, Liu Q. Knockdown of Poc1b causes abnormal photoreceptor sensory cilium and vision impairment in zebrafish. Biochem Biophys Res Commun. 2015;465(4):651–7.
Durlu YK, Koroglu C, Tolun A. Novel recessive cone-rod dystrophy caused by POC1B mutation. JAMA Ophthalmol. 2014;132(10):1185–91.
Roosing S, Lamers IJ, de Vrieze E, van den Born LI, Lambertus S, Arts HH, Peters TA, Hoyng CB, Kremer H, Hetterschijt L, Letteboer SJ. Disruption of the basal body protein POC1B results in autosomal-recessive cone-rod dystrophy. Am J Hum Genet. 2014;95(2):131–42.
Osborn DP, Roccasecca RM, McMurray F, Hernandez-Hernandez V, Mukherjee S, Barroso I, Stemple D, Cox R, Beales PL, Christou-Savina S. Loss of FTO antagonises Wnt signaling and leads to developmental defects associated with ciliopathies. PLoS One. 2014;9(2):e87662.
Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26(2):216–20.
Eisen JS, Smith JC. Controlling morpholino experiments: don’t stop making antisense. Development. 2008;135(10):1735–43.
Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243(2):209–14.
Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491(7422):114–8.
Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci USA. 2013;110(34):13904–9.
Carlson DF, Fahrenkrug SC, Hackett PB. Targeting DNA With Fingers and TALENs. Mol Ther Nucleic Acids. 2012;1:e3.
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308.
Ablain J, Durand EM, Yang S, Zhou Y, Zon LI. A CRISPR/Cas9 vector system for tissue-specific gene disruption in zebrafish. Dev Cell. 2015;32(6):756–64.
Talbot JC, Amacher SL. A streamlined CRISPR pipeline to reliably generate zebrafish frameshifting alleles. Zebrafish. 2014;11(6):583–5.
Simms R, Hynes A, Eley L, Inglis D, Chaudhry B, Dawe H, Sayer J. Modelling a ciliopathy: ahi1 knockdown in model systems reveals an essential role in brain, retinal, and renal development. Cell Mol Life Sci. 2012;69(6):993–1009.
Lim ET, Liu YP, Chan Y, Tiinamaija T, Karajamaki A, Madsen E, Go TDC, Altshuler DM, Raychaudhuri S, Groop L, et al. A novel test for recessive contributions to complex diseases implicates Bardet-Biedl syndrome gene BBS10 in idiopathic type 2 diabetes and obesity. Am J Hum Genet. 2014;95(5):509–20.
Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38(5):521–4.
Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, et al. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80(1):1–11.
Al-Hamed MH, van Lennep C, Hynes AM, Chrystal P, Eley L, Al-Fadhly F, El Sayed R, Simms RJ, Meyer B, Sayer JA. Functional modelling of a novel mutation in BBS5. Cilia. 2014;3(1):3.
Nishimura DY, Baye LM, Perveen R, Searby CC, Avila-Fernandez A, Pereiro I, Ayuso C, Valverde D, Bishop PN, Manson FD, et al. Discovery and functional analysis of a retinitis pigmentosa gene, C2ORF71. Am J Hum Genet. 2010;86(5):686–95.
Joo K, Kim CG, Lee MS, Moon HY, Lee SH, Kim MJ, Kweon HS, Park WY, Kim CH, Gleeson JG, et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci USA. 2013;110(15):5987–92.
Slaats GG, Ghosh AK, Falke LL, Le Corre S, Shaltiel IA, van de Hoek G, Klasson TD, Stokman MF, Logister I, Verhaar MC, et al. Nephronophthisis-associated CEP164 regulates cell cycle progression, apoptosis and epithelial-to-mesenchymal transition. PLoS Genet. 2014;10(10):e1004594.
Baye LM, Patrinostro X, Swaminathan S, Beck JS, Zhang Y, Stone EM, Sheffield VC, Slusarski DC. The N-terminal region of centrosomal protein 290 (CEP290) restores vision in a zebrafish model of human blindness. Hum Mol Genet. 2011;20(8):1467–77.
Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38(6):674–81.
Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, et al. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat Genet. 2012;44(2):193–9.
Wood JD, Bonath F, Kumar S, Ross CA, Cunliffe VT. Disrupted-in-schizophrenia 1 and neuregulin 1 are required for the specification of oligodendrocytes and neurones in the zebrafish brain. Hum Mol Genet. 2009;18(3):391–404.
Maurya AK, Ben J, Zhao Z, Lee RT, Niah W, Ng AS, Iyu A, Yu W, Elworthy S, van Eeden FJ, et al. Positive and negative regulation of Gli activity by Kif7 in the zebrafish embryo. PLoS Genet. 2013;9(12):e1003955.
Tay SY, Ingham PW, Roy S. A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development. 2005;132(4):625–34.
Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40(4):443–8.
Nishiguchi KM, Tearle RG, Liu YP, Oh EC, Miyake N, Benaglio P, Harper S, Koskiniemi-Kuendig H, Venturini G, Sharon D, et al. Whole genome sequencing in patients with retinitis pigmentosa reveals pathogenic DNA structural changes and NEK2 as a new disease gene. Proc Natl Acad Sci USA. 2013;110(40):16139–44.
Dauber A, Lafranchi SH, Maliga Z, Lui JC, Moon JE, McDeed C, Henke K, Zonana J, Kingman GA, Pers TH, et al. Novel microcephalic primordial dwarfism disorder associated with variants in the centrosomal protein ninein. J Clin Endocrinol Metab. 2012;97(11):E2140–51.
Dona M, Bachmann-Gagescu R, Texier Y, Toedt G, Hetterschijt L, Tonnaer EL, Peters TA, van Beersum SE, Bergboer JG, Horn N, et al. NINL and DZANK1 co-function in vesicle transport and are essential for photoreceptor development in zebrafish. PLoS Genet. 2015;11(10):e1005574.
Novorol C, Burkhardt J, Wood KJ, Iqbal A, Roque C, Coutts N, Almeida AD, He J, Wilkinson CJ, Harris WA. Microcephaly models in the developing zebrafish retinal neuroepithelium point to an underlying defect in metaphase progression. Open Biol. 2013;3(10):130065.
Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA. 2011;108(7):2759–64.
Shu X, Zeng Z, Gautier P, Lennon A, Gakovic M, Cheetham ME, Patton EE, Wright AF. Knockdown of the zebrafish ortholog of the retinitis pigmentosa 2 (RP2) gene results in retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):2960–6.
Mahuzier A, Gaude HM, Grampa V, Anselme I, Silbermann F, Leroux-Berger M, Delacour D, Ezan J, Montcouquiol M, Saunier S, et al. Dishevelled stabilization by the ciliopathy protein Rpgrip1 l is essential for planar cell polarity. J Cell Biol. 2012;198(5):927–40.
Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41(6):739–45.
Nair S, Lindeman RE, Pelegri F. In vitro oocyte culture-based manipulation of zebrafish maternal genes. Dev Dyn. 2013;242(1):44–52.
Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42(10):840–50.
Chen Y, Wu B, Xu L, Li H, Xia J, Yin W, Li Z, Shi D, Li S, Lin S, et al. A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res. 2012;22(2):333–45.
Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S, et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31(2):135–40.
Pfaff KL, Straub CT, Chiang K, Bear DM, Zhou Y, Zon LI. The zebra fish cassiopeia mutant reveals that SIL is required for mitotic spindle organization. Mol Cell Biol. 2007;27(16):5887–97.
Chakarova CF, Khanna H, Shah AZ, Patil SB, Sedmak T, Murga-Zamalloa CA, Papaioannou MG, Nagel-Wolfrum K, Lopez I, Munro P, et al. TOPORS, implicated in retinal degeneration, is a cilia-centrosomal protein. Hum Mol Genet. 2011;20(5):975–87.
Poss KD, Nechiporuk A, Stringer KF, Lee C, Keating MT. Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes Dev. 2004;18(13):1527–32.
Authors’ contributions
RM and DO planned, researched, and wrote the paper. Both authors read and approved the final manuscript.
Acknowledgements
RM is supported by the Imperial College London professional and personal development bursary. DO is supported by Fight for Sight UK Charity. We thank the hard work of those research groups cited in this article and apologize to those, which due to word limitations, were not mentioned.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Marshall, R.A., Osborn, D.P.S. Zebrafish: a vertebrate tool for studying basal body biogenesis, structure, and function. Cilia 5, 16 (2016). https://doi.org/10.1186/s13630-016-0036-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13630-016-0036-2