Abstract
Thyroid hormone (TH) signaling plays critical roles in the differentiation, growth, metabolism, and physiological function of all organs or tissues, including heart and skeletal muscle. Due to the significant progress in our understanding of the molecular mechanisms that underlie TH action, it’s widely accepted that TH signaling is regulated at multiple levels. A growing number of discoveries suggest that microRNAs (miRNAs) act as fine-tune regulators of gene expression and adds sophisticated regulatory tiers to signaling pathways. Recently, some pioneering studies in cardiac and skeletal muscle demonstrating the interplay between miRNAs and TH signaling suggest that miRNAs might mediate and/or modulate TH signaling. This review presents recent advances involving the crosstalk between miRNAs and TH signaling and current evidence showing the importance of miRNA in TH signaling with particular emphasis on the study of muscle-specific miRNAs (myomiRs) in cardiac and skeletal muscle. Although the research of the reciprocal regulation of miRNAs and TH signaling is only at the beginning stage, it has already contributed to our current understanding of both TH action and miRNA biology. We also encourage further investigations to address the relative contributions of miRNAs in TH signaling under physiological and pathological conditions and how a group of miRNAs are coordinated to integrate into the complex hierarchical regulatory network of TH.
Similar content being viewed by others
Background
Thyroid hormone
Thyroid, the largest endocrine organ of human body, synthesizes and secretes thyroid hormones (THs), including triiodothyronine (T3) and thyroxine (T4), which act upon nearly every cell in the body. The production of TH by thyroid is tightly controlled by a negative feedback loop of hypothalamus–pituitary–thyroid axis [1–3]. T4 is the most abundant TH in the blood, which is converted into T3, the biologically active form of TH, by iodothyronine deiodinase selenoenzymes in cells. The physiological roles of TH have been extensively studied for more than a century. It is known that TH plays critical roles in growth, differentiation, development, and metabolism [2, 4–6]. One of the most important questions in the field of TH action is how the diverse biological activities of TH are achieved.
Thyroid hormone receptor
The action of TH is mediated mainly through its binding to thyroid hormone receptor (TR), which is a ligand-dependent transcription factor that belongs to the nuclear receptor superfamily [3]. TR binds to the thyroid hormone response element (TRE) within the promoter of target genes as either homodimers or heterodimers with retinoid X receptor (RXR). The transcription mediated by TR involves the T3 binding-induced conformational change of TR and the dynamic interplay with nuclear receptor coregulators [1, 3]. In general, unliganded TR interacts with corepressors to inhibit target gene transcription, while, in the presence of T3, T3-bound TR recruits coactivators to promote the transcription [1]. In contrast, the mechanisms underlying T3-mediated transcriptional repression are still poorly understood. TRs are encoded by two genes, TRα and TRβ, which generate multiple isoforms through alternative splicing or promoter. Among these TRs, only TRα1 and TRβ1-3 have the capacity to bind T3 [7]. TRα and TRβ genes are differentially expressed during development and in adult tissues, which provides an additional means to modulate the TH actions in a temporal and spatial manner [3, 7]. In addition, TR is subject to posttranslational modification by phosphorylation and sumoylation, which also contribute to the modulation of TR activity [8, 9].
Thyroid hormone and its action on cardiac and skeletal muscle
Effects of thyroid hormone on heart
The close relationship between thyroid and heart was noted in the earliest descriptions of hyperthyroidism. The impact of thyrotoxicosis on cardiovascular system was first described in 1785 by Caleb Parry, a British physician who noticed an association between a swelling neck and heart failure [10]. In general, the profound effects of TH on cardiac function include enhancing overall total protein synthesis, lowering systemic vascular resistance, increasing blood volume, and modulating inotropic and chronotropic response [11]. The combination of these effects on both the circulation and the heart itself results in an increase in cardiac output. Hyperthyroid patients exhibit a high cardiac output state, whereas hypothyroid patients display low cardiac output, decreased stroke volume, decreased vascular volume, and increased systemic vascular resistance [11, 12]. It is well accepted that both the direct regulation of target genes by TH and the indirect effects of TH on hemodynamics contribute to these changes [11–13].
Myosin is one of the key components of contractile apparatus, where chemical energy of adenosine triphosphate (ATP) is converted to mechanical work [14]. There are three isoenzymes of ventricular myosin in heart, which differ in their myosin heavy chain (MHC) α and β composition: myosin V1 (α/α), myosin V2 (α/β), and myosin V3 (β/β) [14]. Myosin V1 has the highest ATPase activity and V3 the lowest. Myosin V2 has an ATPase activity intermediate between V1 and V3. Thus, the ratio of the V1 to the V3 isoenzyme of myosin determines cardiac contractility and correlates with cardiac muscle performance as indicated by mechanical, myothermal, and biological analysis [14]. The relative amounts of the three isoforms (V1, V2, and V3) not only change with age, or stress (exercise or ventricular pressure overload), but also change under altered thyroid states (hypothyroidism or hyperthyroidism) [15].
It is known that TH has opposing effects on α-MHC and β-MHC gene expression [16, 17]. Putative TREs for both α-MHC and β-MHC genes have been reported in the promoter region of these two genes. The two TREs identified in the promoter of α-MHC are imperfect direct repeats, which are separated by four nucleotides [18, 19]. Regarding β-MHC, a putative negative TRE (nTRE) containing a single half-site is found adjacent to the TATA box [20, 21]. The increase of α-MHC gene expression and the decrease of β-MHC gene expression by T3 result in an increase in myosin V1 levels and enhanced cardiac contractility [19]. In contrast, induction of a hypothyroid state increases myosin V3 composition, which leads to a decrease in velocity of fiber shortening [16, 19]. In addition, a surge in TH levels after birth also contributes to the developmental switch in the ventricles of rat, in which α-MHC mRNA increases shortly after birth and almost replaces β-MHC mRNA completely in a week [22]. Although the developmental pattern for MHC isoforms is different and the effect of T3 on α-MHC is small in higher mammals, a clinical study showed that T4 replacement improved cardiac function of a hypothyroid patient, which was accompanied with a more than 10-fold increase of α-MHC mRNA levels in the patient’s ventricles [23]. Therefore, due to its ability to control the expression of MHC isoforms, TH is a critical player in the regulation of cardiac function.
Changing concentrations of cytosolic Ca2+ in cardiac myocytes controls each cycle of contraction and relaxation [24]. Cardiac sarcoplasmic reticulum calcium ATPase (SERCA2), which is an intracellular ion pump, plays a critical role in maintaining the intracellular Ca2+ homeostasis [25]. SERCA2 removes the Ca2+ from the cytosol and stores the Ca2+ in the sarcoplasmic reticulum, which leads to diastolic relaxation [25]. The expression of SERCA2 was decreased in hypothyroid rats, while the levels of SERCA2 could be elevated by T3 treatment, suggesting that SERCA2 is positively regulated by TH [26, 27]. Thus, TH is able to relax the heart and enhance cardiac output by reducing the amount of cytosolic Ca2+ through increasing SERCA2 expression [26, 27]. For the time being, three TREs arranged as direct repeats (DRs) and inverted palindromes have been identified in the promoter region of SERCA2 gene [28]. Besides regulating SERCA2 expression, T3 can also shape heart function by targeting other ion channels such as voltage-gated potassium (Kv) channel Kv1.5, Na+/K+-ATPase, and hyperpolarization activated cyclic nucleotide-gated channel [29, 30]. In addition, TH may enhance the cardiac sensitivity to catecholamines through controlling the β-adrenergic receptor expression [31].
Recent findings from TR mutant mouse models substantiate the role of TH in the physiology of heart [32]. Introducing a dominant negative mutant TRβ into the heart of mice suppressed the mRNA expression of α-MHC and SERCA2, but upregulated the mRNA levels of β-MHC, which led to prolonged cardiac muscle contraction and QRS interval [33]. Studies using TR isoform-specific mouse models have elucidated that TRα and TRβ play differential roles in the regulation of heart rate [34]. Mice deficient in TRα showed decreased heart rate and prolonged QRS interval, whereas mice lacking TRβ had elevated heart rate, which was resistant to TH treatment. These findings suggest that TRα1 is more important for maintaining baseline heart rate, whereas TRβ may be only involved in TH-mediated stimulation of heart rate [34, 35]. Due to the beneficial effect of TH on cardiac function, T3 has been employed for heart surgery including cardiac transplantation and cardiac bypass surgery.
Effects of thyroid hormone on skeletal muscle
Skeletal muscle is a major target organ of TH. Myopathic symptoms are very common among hyperthyroid or hypothyroid patients [36]. The effects of THs on muscle contractility and metabolism have been extensively studied [5]. It is known that MyHC gene expression is controlled by TH [16, 37, 38]. Muscles of hypothyroid patients typically display a conversion from fast to slow fiber types, and a more efficient energy metabolism [39]. Hypothyroid patients have less type-II fibers in the muscle, as compared to euthyroid patients [39]. Interestingly, hypothyroid females have higher proportion of type-II fibers than hypothyroid males; however, type-II fiber atrophy occurs only in hypothyroid female patients [40], suggesting there is a gender-dependent mechanism involved.
In general, experimental hyperthyroidism in rats using T3 could induce a reversible slow-to-fast MyHC isoform transition from I → IIa → IIx → IIb [41]. However, gender- and muscle-specific differences were always observed in the regulation of MyHC isoforms by T3 in rats. Normally, almost all myofibers in the soleus muscle of rats expressed the slow MyHC isoform (type-I fibers) [38]. Four weeks of T3 treatment resulted in an increase of type-IIA fibers and a decrease of type-I fibers [42]. After T3 treatment, compared to male rats, the soleus muscle of female rats expressed more type-I fibers and less type-I/IIa and type-I/IIax fibers [39]. Moreover, the IIx content from the type-I/IIax fibers increased more in T3-treated male rats than that in female rats, while the upregulation of IIa content was greater in female rats than that in male rats [39]. In addition, it has been reported that T3 treatment induced IIx MyHC isoform expression only in the soleus muscle of male rats, whereas IIx expression could not be detected in female rats at any age [43].
In contrast to soleus muscles, extensor digitorum longus (EDL) muscles contain predominantly fast MyHC isoforms (IIa, IIx, and IIb) [44]. Increased levels of MyHC-IIa were observed in the EDL muscle of hypothyroid rat [45]. One study showed that long-term T3 treatment reduced both MyHC-IIb and MyHC-IIa expression at mRNA and protein levels in the EDL muscle [46]. However, in another study, chronic hyperthyroidism increased MyHC-IIb mRNA expression without changing the protein levels in rat EDL muscle [47]. It has been reported that T3 treatment in euthyroid rats resulted in a transition from IIa to IIb fibers only in the EDL muscle of female rats, but not in that of male rats, further suggesting there is a gender difference in fiber-type conversion [40]. These studies indicate that the effect of TH on muscle contractibility, endurance, and the response to fatigue might differ between females and males, presumably as a result of a gender difference in the regulation of fiber-type switch.
MicroRNAs biogenesis and functions
MicroRNAs (miRNAs), first identified in C. elegans in the early 1900s [48], are a group of highly conserved noncoding RNAs and approximately 22 nucleotides in length [49]. miRNAs function primarily as negative regulators of gene expression at the post-transcriptional level [49, 50]. miRNAs can be classified as intronic and intergenic based on their genomic location [49, 51]. In general, intronic miRNAs are located in the introns of protein coding genes and transcribed along with their host genes, while intergenic miRNAs have their own promoters and are transcribed as independent transcripts [49, 51].
miRNAs are usually transcribed by RNA polymerase II as primary miRNAs (pri-miRNAs) in the nucleus [52]. However, it also has been reported that a few miRNAs requires RNA polymerase III to generate pri-miRNAs [53]. After transcription, pri-miRNAs are then processed by microprocessor complex, which is composed of two core components, dsRNA-binding protein DGCR8 and RNase III endonuclease Drosha [52, 54]. Microprocessor complex binds to the stem-loop structure of pri-miRNAs and cleaves the primary transcripts to release a hairpin-shaped RNA molecule known as precursor miRNAs (pre-miRNAs) [52, 54]. The double-stranded pre-miRNAs are 70–100 nucleotides in length, and subsequently transported from nucleus to the cytoplasm by Exportin-5 for further processing [52, 54]. Dicer, which is a RNase III endonuclease and responsible for the maturation of miRNAs in the cytoplasm, cleaves pre-miRNA to generate a double stranded miRNA duplex (miRNA–miRNA*) with 20–25 nucleotides in length [52, 54, 55]. The mature miRNA duplex is then recognized by the RNA induced silencing complex (RISC) containing Dicer and AGO2 (argonaute RISC catalytic component 2). Usually, only one strand of miRNA duplex is preferentially chosen to be incorporated into the RISC to form miRNA-induced silencing complex (miRISC) [55, 56]. The miRNA-loaded RISC binds to the target mRNAs and silences the gene expression through either degradation of mRNA or inhibition of translation [55, 56].
MiRNA and thyroid hormone signaling
TH signaling has profound effects on many physiologic processes. The effectiveness of TH signaling relies on their capacity to tightly control the expression of target genes in time and space. The temporal and spatial activities of TH could be achieved through the regulation of the systemic and local levels of TH. The circulating TH levels are exquisitely regulated by a negative-feedback system that involves the hypothalamus–pituitary–thyroid axis, while the intracellular TH levels are modulated by type 2 deiodinase, which is responsible for the conversion of prohormone T4 to bioactive T3. Recently, the miRNA field has grown tremendously and emerging evidence suggests that miRNAs not only confer signaling robustness as amplifiers, balancers, or buffers but also play important roles in signaling crosstalk and coordination as nodes of signaling networks. In 2007, Olson’s group published a research article in Science magazine that first linked the role of miRNA to TH signaling [57]. Soon afterward, the possible involvement of miRNAs in TH signaling networks has been indicated by several studies in a variety of model systems. In this review we summarize the major research progress with an emphasis on muscle-specific miRNAs (myomiRs), including miR-208a/b, miR-499, miR-133, which play important roles in the regulation of the development, plasticity, and health of mammalian skeletal and cardiac muscles.
MiR-208a
MiR-208a is located in the intron of α-MHC gene, which encodes a major cardiac contractile protein [58]. It was shown that cardiac-specific miR-208a was required for cardiomyocyte hypertrophy, fibrosis, and the expression of β-MHC in response to hypothyroidism [57]. Ablation of miR-208a attenuated cardiac hypertrophy in response to pressure overload and reduced β-MHC expression [57]. While cardiac overexpression of miR-208a was sufficient to induce cardiac hypertrophy and cardiac conduction abnormalities [57]. Further study showed that miR-208a acted through repressing the TR co-regulator THRAP1 and myostatin, two negative regulators of muscle growth and hypertrophy [57, 58]. Accordingly, inhibition of miR-208a by antisense oligonucleotide delivery improved cardiac function, overall health, and survival during hypertension-induced heart failure [59].
It is well established that TH promotes α-MHC and inhibits β-MHC expression in the heart [16, 17]. Similar to the regulation of α-MHC by TH, the expression of miR-208a is also significantly blunted in the adult heart in response to hypothyroidism. To further explore the roles of miR-208a in the cardiac muscle, β-MHC expression was investigated in miR-208a knockout mice treated with propylthiouracil (PTU), which rendered the mice hypothyroid [58]. Interestingly, hypothyroidism could not induce β-MHC expression in the heart of miR-208a null mice pathway [58]. These findings provided direct evidence that miR-208a mediates TH action in heart.
MiR-208b and miR-499
Besides miR-208a, miR-208 family contains another two miRNAs, miR-208b and miR-499. They are encoded in different myosin genes, Myh7 (β-MHC) and Myh7b, respectively [60, 61]. These two intronic miRNAs have been shown to be involved in the control of muscle fiber type by activating slow and repressing fast myofiber gene programs [62]. Recently, it was reported that miR-208b and miR-499 are downstream targets of estrogen-related receptor γ (ERRγ), which mediates the effect of peroxisome proliferators-activated receptors β/δ and PPARα on energy metabolism and skeletal muscle fiber specificity [63, 64]. As a result, activation of ERRγ could lead to an upregulation of miR-208b/miR-499 and subsequently increases type I muscle fiber proportion in skeletal muscle [65]. As mentioned early, it is known that skeletal muscle is a target for TH and all members of the MyHC family respond to TH. Given that TH regulates myofiber type transition [66], it is not surprising to find that hypothyroid state induces both Myh7/miR-208b and Myh7b/miR-499 expression in skeletal muscle [62]. These findings indicate that miR-208b and miR-499 might mediate the effect of TH on myofiber type determination and energy metabolism.
MiR-133
MiR-133 family is one of the most studied miRNA families in skeletal muscle [67]. The enrichment of miR-133 as well as miR-1 and miR-206 in heart and skeletal muscle was first reported in 2004 [68], which was confirmed subsequently by several groups. Due to their tissue specific expression, these miRNAs are designated as canonical myomiRs, which have been extensively investigated [67, 69–72]. The miR-133 family (including miR-133a1, miR-133a and miR-133b) and miR-1 family (including miR-1-1, miR-1-2 and miR-206) are clustered on human chromosomes. Their sequences and expressions are evolutionarily conserved across species [73].
Further study showed that miR-133a is highly expressed both in heart and in skeletal muscle, while miR-133b is specifically expressed in skeletal muscle [74]. Several studies confirmed that miR-133a played a regulatory role in the development of cardiac and skeletal muscle [72, 75, 76]. The most direct evidence came from miR-133a1 and miR-133a2 knockout mouse models. In the heart, absence of miR-133a resulted in ectopic expression of smooth muscle genes and aberrant cardiomyocyte proliferation due to elevated expression of SRF and cyclin D2, which were direct targets of miR-133a [72]. In the skeletal muscle, miR-133a knockout mice developed adult-onset centro nuclear myopathy in type II (fast-twitch) myofiber, accompanied by fast-to-slow myofiber transition, suggesting an essential role of miR-133a in maintaining the structure, function, and myofiber types of skeletal muscle [77].
The link between TH and myomiRs was reported in flounder (Paralichthys olivaceus), which showed that miR-133a as well as miR-1 and miR-206a were TH-regulated miRNAs during larval development [78]. By using hypo- and hyperthyroid mouse models and TR isoform–specific knockout mice, we demonstrated that miR-133a1 transcription could be up-regulated directly by TH in a TR-dependent manner [38]. Our study also showed for the first that overexpression of miR-133a was able to promote slow-to-fast muscle switch by repressing TEA domain family member 1 (TEAD1), a key modulator of slow muscle gene [38]. Based on our in vivo and in vitro data, we proposed that miR-133a1 mediates the effect of TH on muscle fiber type specification [38]. Furthermore, our data showed that the negative regulation of MyHC-I transcription by TH was indirect, suggesting that TH might not regulate MyHC-I transcription through a nTRE [38]. In addition, since miR-133a not only regulates myofiber type switch but also plays a role in proliferation, regeneration and remodeling, we speculated that TH signaling might have a variety of effects on muscle physiology and pathology through miR-133a1-mediated mechanisms.
Other miRNAs
Besides myomiRs, a few other miRNAs have been shown to serve as either regulators or components of TH signaling. For example, miR-27a was demonstrated to modulate β-MHC gene expression in cardiomyocytes [79]. In cardiac hypertrophy and skeletal muscle atrophy, miR-27a was strongly increased via Srf and Myf6 transactivation [80, 81]. MiR-27a was upregulated during ES cell differentiation and heart development in mouse, implying a critical role of miR-27a in heart development [79]. Moreover, miR-27a overexpression strongly upregulated the β-MHC, but not α-MHC by targeting TRβ1 [79]. These studies highlighted the role of miR-27a in cardiac pathophysiology as a regulator in the TH signaling pathway.
MiR-30a belongs to the miR-30 family, which is highly expressed in the heart [82]. Previous studies have shown that members of miR-30 family were downregulated in cardiomyocytes in response to ROS [83]. And they also play critical roles in calcium/calcineurin signaling in cardiomyocytes, which was demonstrated by delivering a miR-30 sponge to cardiomyocytes [84]. A recent study revealed that T3 could improve the recovery of post-ischemic cardiac performance via regulating the miR-30a/p53 axis [85]. In the ischemia/reperfusion (I/R) injury model, T3 could restore the expression level of miR-30a and then prevent the upregulation of p53, suggesting an important role of miR-30a in mediating the cardioprotective effects of T3 [85].
Type 3 deiodinase (Dio3) is a TH-inactivating enzyme usually expressed at fetal stage, which regulates proliferation. In a myocardial infarction (MI) mouse model, Dio3 is up-regulated in cardiomyocytes to create a local hypothyroid condition to increase the regenerative capacity by initiating the fetal gene program. A group of miRNAs with altered expression levels were identified in this MI model and were predicted to be involved in the regulation of stress response in cardiomyocytes [86]. Further study showed that Dio3 is a target of miR-214 [87]. Therefore, the upregulation of miR-214 observed in MI model might dampen the MI-induced upregulation of Dio3. The finding that miR-214 was negatively regulated by TH suggests that miR-214 and Dio3 form a negative feedback loop in cardiomyocytes. The interplay between miR-214 and Dio3 after MI provides a mechanism to protect the adult cardiomyocyte from the adverse effect of local TH deficiency [87].
It is known that hyperthyroidism induces cardiac hypertrophy and the Angiotensin type 1 receptor (AT1R) has been demonstrated to mediate part of this response. It was shown that T3 treatment increased AT1R mRNA and protein levels rapidly in cardiomyocyte, which was accompanied with an increased of miR-350 expression. Since AT1R is one of predicted target of miR-350, it is reasonable to make the assumption that miR-350 might be involved in TH-induced cardiomyocyte hypertrophy [88]. On the other hand, two studies indicate that AT1R might contribute to the altered expression of cardiac miR-208a and miR-133 induced by hyperthyroidism [89, 90]. These findings provide new insights in the understanding of regulatory networks involved in the cardiac growth controlled by miRNAs and TH signaling.
Perspective
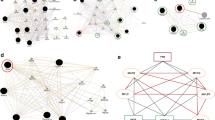
The effects of TH on heart and muscle have been well established. The emergence of the miRNA field has provided a unique avenue towards deeper understanding of the TH action in cardiac and skeletal muscle development, regeneration and physiology. Recent studies involving miRNAs and TH signaling in cardiac and skeletal muscle have shed light on the contributions of miRNAs to TH signaling (Fig. 1). These studies suggest that miRNAs have added an additional layer of complexity to the extensive TH effects in normal and disease states. Moreover, certain miRNAs have been proved to be the missing links to previously unrecognized mechanisms. So far, since most studies have tried to pinpoint the mechanism of miRNAs to their single downstream targets, systems biology approaches might be a better choice to explain miRNA activity under euthyroid, hyper and hypothyroid conditions. Despite the uncertainties, some of these miRNAs have the potential to eventually become heart or muscle disease biomarkers or even drug targets in future. Further investigations are required to address the relative contributions of miRNAs under physiological and pathological conditions and how a group of miRNAs are coordinated to integrate into the complex hierarchical regulatory network of TH.
Abbreviations
- TH:
-
thyroid hormone
- miRNAs:
-
microRNAs
- MyomiRs:
-
muscle-specific miRNAs
- T3:
-
triiodothyronine
- T4:
-
thyroxine
- TR:
-
thyroid hormone receptor
- TRE:
-
thyroid hormone response element
- RXR:
-
retinoid X receptor
- ATP:
-
adenosine triphosphate
- MHC:
-
myosin heavy chain
- nTRE:
-
negative TRE
- SERCA2:
-
sarcoplasmic reticulum calcium ATPase
- DRs:
-
direct repeats
- IPs:
-
inverted palindromes
- Kv:
-
voltage-gated potassium
- HCN:
-
hyperpolarization activated cyclic nucleotide-gated
- pri-miRNAs:
-
primary miRNAs
- pre-miRNAs:
-
precursor miRNAs
- RISC:
-
RNA induced silencing complex
- miRISC:
-
miRNA-induced silencing complex
- AGO2:
-
argonaute RISC catalytic component 2
- PTU:
-
propylthiouracil
- ERRγ:
-
estrogen-related receptor γ
- TEAD1:
-
TEA domain family member 1
- I/R:
-
ischemia/reperfusion
- Dio3:
-
type 3 deiodinase
- MI:
-
myocardial infarction
- AT1R:
-
Angiotensin type 1 receptor
References
Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–142.
Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–82.
Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847–53.
Kress E, Samarut J, Plateroti M. Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol. 2009;313:36–49.
Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid. 2008;18:205–16.
Thompson CC, Potter GB. Thyroid hormone action in neural development. Cereb Cortex. 2000;10:939–45.
O’Shea PJ, Williams GR. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J Endocrinol. 2002;175:553–70.
Jeyakumar M, Liu XF, Erdjument-Bromage H, Tempst P, Bagchi MK. Phosphorylation of thyroid hormone receptor-associated nuclear receptor corepressor holocomplex by the DNA-dependent protein kinase enhances its histone deacetylase activity. J Biol Chem. 2007;282:9312–22.
Liu YY, Ayers S, Milanesi A, Teng XC, Rabi S, Akiba Y, Brent GA. Thyroid hormone receptor sumoylation is required for preadipocyte differentiation and proliferation. J Biol Chem. 2015;290:7402–15.
Hull G. Caleb Hillier Parry 1755-1822: a notable provincial physician. J R Soc Med. 1998;91:335–8.
Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–28.
Sabatino L, Iervasi G, Pingitore A. Thyroid hormone and heart failure: from myocardial protection to systemic regulation. Expert Rev Cardiovasc Ther. 2014;12:1227–36.
Li M, Iismaa SE, Naqvi N, Nicks A, Husain A, Graham RM. Thyroid hormone action in postnatal heart development. Stem Cell Res. 2014;13:582–91.
Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest. 1997;100:2362–70.
Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Arch Med Sci. 2013;9:944–52.
Haddad F, Jiang W, Bodell PW, Qin AX, Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol. 2010;299:H1968–80.
Gustafson TA, Markham BE, Morkin E. Effects of thyroid-hormone on alpha-actin and myosin heavy-chain gene-expression in cardiac and skeletal-muscles of the rat—measurement of messenger-RNA content using synthetic oligonucleotide probes. Circ Res. 1986;59:194–201.
Chiamolera MI, Wondisford FE. Minireview: thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–6.
Morkin E. Control of cardiac myosin heavy chain gene expression. Microsc Res Tech. 2000;50:522–31.
Wright CE, Haddad F, Qin AX, Bodell PW, Baldwin KM. In vivo regulation of beta-MHC gene in rodent heart: role of T3 and evidence for an upstream enhancer. Am J Physiol. 1999;276:C883–91.
Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 1985;2001(90):345–57.
Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol. 2007;43:388–403.
Mourouzis I, Forini F, Pantos C, Iervasi G. Thyroid hormone and cardiac disease: from basic concepts to clinical application. J Thyroid Res. 2011;2011:958626.
Fearnley CJ, Roderick HL, Bootman MD. Calcium signaling in cardiac myocytes. Cold Spring Harb Perspect Biol. 2011;3:a004242.
Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2 + ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–73.
Ojamaa K. Signaling mechanisms in thyroid hormone-induced cardiac hypertrophy. Vascul Pharmacol. 2010;52:113–9.
Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15:125–32.
Velasco LF, Togashi M, Walfish PG, Pessanha RP, Moura FN, Barra GB, Nguyen P, Rebong R, Yuan C, Simeoni LA, et al. Thyroid hormone response element organization dictates the composition of active receptor. J Biol Chem. 2007;282:12458–66.
Abe A, Yamamoto T, Isome M, Ma M, Yaoita E, Kawasaki K, Kihara I, Aizawa Y. Thyroid hormone regulates expression of shaker-related potassium channel mRNA in rat heart. Biochem Biophys Res Commun. 1998;245:226–30.
Nishiyama A, Kambe F, Kamiya K, Seo H, Toyama J. Effects of thyroid status on expression of voltage-gated potassium channels in rat left ventricle. Cardiovasc Res. 1998;40:343–51.
Hoit BD, Khoury SF, Shao Y, Gabel M, Liggett SB, Walsh RA. Effects of thyroid hormone on cardiac beta-adrenergic responsiveness in conscious baboons. Circulation. 1997;96:592–8.
Johansson C, Vennstrom B, Thoren P. Evidence that decreased heart rate in thyroid hormone receptor-alpha 1-deficient mice is an intrinsic defect. Am J Physiol Regul Integ Comp Physiol. 1998;275:R640–6.
Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circu Physiol. 2000;278:H412–9.
Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70.
Johansson C, Gothe S, Forrest D, Vennstrom B, Thoren P. Cardiovascular phenotype and temperature control in mice lacking thyroid hormone receptor-beta or both alpha(1) and beta. Am J Physiol Heart Circ Physiol. 1999;276:H2006–12.
Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry. 2000;68:750–5.
Brent GA, Moore DD, Larsen PR. Thyroid hormone regulation of gene expression. Annu Rev Physiol. 1991;53:17–35.
Zhang D, Wang X, Li Y, Zhao L, Lu M, Yao X, Xia H, Wang YC, Liu MF, Jiang J, et al. Thyroid hormone regulates muscle fiber type conversion via miR-133a1. J Cell Biol. 2014;207:753–66.
Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30:30–9.
McKeran RO, Slavin G, Andrews TM, Ward P, Mair WG. Muscle fibre type changes in hypothyroid myopathy. J Clin Pathol. 1975;28:659–63.
Larsson L, Li X, Teresi A, Salviati G. Effects of thyroid hormone on fast- and slow-twitch skeletal muscles in young and old rats. J Physiol. 1994;481(Pt 1):149–61.
Simonides WS, van der Linden GC, van Hardeveld C. Thyroid hormone differentially affects mRNA levels of Ca-ATPase isozymes of sarcoplasmic reticulum in fast and slow skeletal muscle. FEBS Lett. 1990;274:73–6.
Larsson L, Yu F. Gender-related differences in the regulatory influence of thyroid hormone on the expression of myosin isoforms in young and old rats. Acta Physiol Scand. 1997;159:81–9.
Soukup T, Zacharova G, Smerdu V. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem. 2002;104:399–405.
Soukup T, Sulimenko V, Markova V, Kopecka K, Zacharova G, Palecek J. Expression of the skeletal calsequestrin isoform in normal and regenerated skeletal muscles and in the hearts of rats with altered thyroid status. Physiol Res. 2012;61:575–86.
Vadaszova A, Hudecova S, Krizanova O, Soukup T. Levels of myosin heavy chain mRNA transcripts and protein isoforms in the fast extensor digitorum longus muscle of 7-month-old rats with chronic thyroid status alterations. Physiol Res. 2006;55:707–10.
Kirschbaum BJ, Kucher HB, Termin A, Kelly AM, Pette D. Antagonistic effects of chronic low frequency stimulation and thyroid hormone on myosin expression in rat fast-twitch muscle. J Biol Chem. 1990;265:13974–80.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11:1753–61.
Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta. 2010;1803:1231–43.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Diebel KW, Smith AL, van Dyk LF. Mature and functional viral miRNAs transcribed from novel RNA polymerase III promoters. RNA. 2010;16:170–85.
Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–27.
Davis BN, Hata A. Regulation of MicroRNA Biogenesis: a miRiad of mechanisms. Cell Commun Signal. 2009;7:18.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24.
van Rooij E, Sutherland LB, Qi XX, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–9.
Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng ZL, Gunn B, Shumate J, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Investig. 2009;119:2772–86.
Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN, van Rooij E. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011;124:1537.
Kakimoto Y, Tanaka M, Kamiguchi H, Hayashi H, Ochiai E, Osawa M. MicroRNA deep sequencing reveals chamber-specific miR-208 family expression patterns in the human heart. Int J Cardiol. 2016;211:43–8.
Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac Injury and protection. Mol Pharmacol. 2011;80:558–64.
van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi XX, Richardson JA, Kelm RJ, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–73.
Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor at signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–91.
Azhar S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010;6:657–91.
Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, Xie H, Conley KE, Auwerx J, Smith SR, et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest. 2013;123:2564–75.
Yu F, Gothe S, Wikstrom L, Forrest D, Vennstrom B, Larsson L. Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1545–54.
Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33.
Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13.
Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5(3):R13.
Zhang D, Li X, Chen C, Li Y, Zhao L, Jing Y, Liu W, Wang X, Zhang Y, Xia H, Chang Y. Attenuation of p38-mediated miR-1/133 expression facilitates myoblast proliferation during the early stage of muscle regeneration. PLoS ONE. 2012;7(7):e41478.
Yang BF, Lin HX, Xiao JN, Lu YJ, Luo XB, Li BX, Zhang Y, Xu CQ, Bai YL, Wang HZ, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2 (vol 13, p 486, 2007). Nat Med. 2011;17:1693.
Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–54.
Mitchelson KR, Qin WY. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J Biol Chem. 2015;6:162–208.
Townley-Tilson WH, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol. 2010;42:1252–5.
Izarra A, Moscoso I, Levent E, Canon S, Cerrada I, Diez-Juan A, Blanca V, Nunez-Gil IJ, Valiente I, Ruiz-Sauri A, et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports. 2014;3:1029–42.
Yin VP, Lepilina A, Smith A, Poss KD. Regulation of zebrafish heart regeneration by miR-133. Dev Biol. 2012;365:319–27.
Liu N, Bezprozvannaya S, Shelton JM, Frisard MI, Hulver MW, McMillan RP, Wu Y, Voelker KA, Grange RW, Richardson JA, et al. Mice lacking microRNA 133a develop dynamin 2-dependent centronuclear myopathy. J Clin Invest. 2011;121:3258–68.
Fu YS, Shi ZY, Wang GY, Li WJ, Zhang JL, Jia L. Expression and regulation of miR-1, -133a, -206a, and MRFs by thyroid hormone during larval development in Paralichthys olivaceus. Comp Biochem Physiol B. 2012;161:226–32.
Nishi H, Ono K, Horie T, Nagao K, Kinoshita M, Kuwabara Y, Watanabe S, Takaya T, Tamaki Y, Takanabe-Mori R, et al. MicroRNA-27a regulates beta cardiac myosin heavy chain gene expression by targeting thyroid hormone receptor beta1 in neonatal rat ventricular myocytes. Mol Cell Biol. 2011;31:744–55.
Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, Guo S, Wang Y, Fan K, Zhan D, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell Res. 2012;22:516–27.
Wang XH. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care. 2013;16:258–66.
Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–8.
Roca-Alonso L, Castellano L, Mills A, Dabrowska AF, Sikkel MB, Pellegrino L, Jacob J, Frampton AE, Krell J, Coombes RC, et al. Myocardial MiR-30 downregulation triggered by doxorubicin drives alterations in beta-adrenergic signaling and enhances apoptosis. Cell Death Dis. 2015;6:e1754.
Wu J, Zheng C, Wang X, Yun S, Zhao Y, Liu L, Lu Y, Ye Y, Zhu X, Zhang C, et al. MicroRNA-30 family members regulate calcium/calcineurin signaling in podocytes. J Clin Invest. 2015;125:4091–106.
Forini F, Kusmic C, Nicolini G, Mariani L, Zucchi R, Matteucci M, Iervasi G, Pitto L. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology. 2014;155:4581–90.
Janssen R, Zuidwijk M, Muller A, Mulders J, Oudejans CB, Simonides WS. Cardiac expression of deiodinase type 3 (Dio3) following myocardial infarction is associated with the induction of a pluripotency microRNA signature from the Dlk1-Dio3 genomic region. Endocrinology. 2013;154:1973–8.
Janssen R, Zuidwijk MJ, Muller A, van Mil A, Dirkx E, Oudejans CB, Paulus WJ, Simonides WS. MicroRNA 214 is a potential regulator of thyroid hormone levels in the mouse heart following myocardial infarction, by targeting the thyroid-Hormone-inactivating enzyme deiodinase type III. Front Endocrinol (Lausanne). 2016;7:22.
Diniz GP, Takano AP, Bruneto E, Silva FG, Nunes MT, Barreto-Chaves ML. New insight into the mechanisms associated with the rapid effect of T(3) on AT1R expression. J Mol Endocrinol. 2012;49:11–20.
Diniz GP, Takano AP, Barreto-Chaves ML. MiRNA-208a and miRNA-208b are triggered in thyroid hormone-induced cardiac hypertrophy—role of type 1 angiotensin II receptor (AT1R) on miRNA-208a/alpha-MHC modulation. Mol Cell Endocrinol. 2013;374:117–24.
Diniz GP, Lino CA, Guedes EC, Moreira Ldo N, Barreto-Chaves ML. Cardiac microRNA-133 is down-regulated in thyroid hormone-mediated cardiac hypertrophy partially via type 1 angiotensin II receptor. Basic Res Cardiol. 2015;110:49.
Authors’ contributions
DZ and HY laid out the review focuses and wrote/revised the manuscript. DZ, FG, QZ, JJ, and HY composed the manuscript outline. YL, SL, YW, and JJ contributed to the literature search for the manuscript. YL, SL, YW, FG, QZ, and JJ all participated in the writing and revision. FG, QZ, and HY did proof reading and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Funding
This work was supported by grants from Ministry of Science and Technology of the People’s Republic of China (2016YFA0500102, 2016YFC1304905), National Natural Science Foundation (31525012, 31500959, 31371189, 81570768, and 81471016), Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBS2012004), CAS/SAFEA International Partnership Program for Creative Research Teams, and Xuhui Central Hospital (Shanghai, China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, D., Li, Y., Liu, S. et al. microRNA and thyroid hormone signaling in cardiac and skeletal muscle. Cell Biosci 7, 14 (2017). https://doi.org/10.1186/s13578-017-0141-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-017-0141-y