Abstract
Conventional skeletal chondrosarcoma is a bone neoplasm, which is poorly sensitive to anthracyclines-based chemotherapy. We report on an 18-month-long tumour response to gemcitabine as single agent in a young patient with an advanced secondary peripheral conventional chondrosarcoma, previously treated unsuccessfully with anthracyclines, ifosfamide, platinum, etoposide.
Similar content being viewed by others
Background
The skeletal chondrosarcoma family represents a heterogeneous group of malignant bone mesenchymal tumours characterised by the production of a chondroid matrix. They are the third bone sarcoma in incidence, and the most frequent in adults. There are three main subtypes: conventional, mesenchymal, and clear-cell. A “dedifferentiated” chondrosarcoma develops in 10-15% of conventional chondrosarcomas, while mesenchymal chondrosarcoma is a high-grade, aggressive neoplasm with a natural history and chemosensitivity that might be close to Ewing sarcoma, and clear-cell chondrosarcoma is a low-grade variant [1].
In conventional chondrosarcoma (cCS), the histological malignancy grade is the main prognostic factor [2]. Grade 1 cCS are characterised by a very low metastatic potential, and some authors have quite recently suggested a re-classification of these types as “atypical cartilaginous tumours” [1]. Grade 2 and 3 cCS are marked by a higher metastatic potential, with a 10-year survival of 64-86% and 29-55% respectively [3,4].
CCSs are also categorised according to their location in the bone: a central chondrosarcoma onsets in the medullary cavity, a small percentage of them from a pre-existing benign lesion known as enchondroma, while a peripheral variant arises from the surface of the bone, as a result of malignant progression of a pre-existing benign (solitary or hereditary) osteochondroma.
Surgery is the mainstay of the treatment of localized disease. While curettage is acceptable for grade 1 cCS, wide excision is usually required for higher grade cCS, with the exception of skull base cCS which may be treated with radiotherapy. In particular, hadrons can play an important role in the management of skull base cCS, and very good outcomes are reported [5].
In surgically treated patients, the benefit of adding radiotherapy and chemotherapy remains unclear, due to a lack of prospective trials. Adjuvant radiotherapy and/or chemotherapy may be proposed to high-risk patients in conditions of uncertainty. When cCS is advanced, and a medical therapy is the only option, regimens commonly used in other bone sarcomas are employed [6]. Traditionally, chemotherapy has been considered poorly effective [7], but the low number of cases and the inclusion in available series of conventional (both central and peripheral), dedifferentiated, mesenchymal, clear-cell histotypes does not help to understand the actual chemo-responsiveness of the disease. Recently, responses to gemcitabine in combination with docetaxel have been reported in advanced chondrosarcomas [8].
Hereby, we describe the case of a young woman with a metastatic, pretreated cCS treated with gemcitabine as a single agent, after failing to anthracyclines, ifosfamide, cisplatin, etoposide.
Case presentation
Patient characteristics and medical history
In December 2009, a 38-year old woman, in good general conditions, was diagnosed a 17-cm large mass arising from an osteochondroma of the left iliac bone (Figure 1). Diagnostic biopsy revealed grade 2 secondary peripheral cCS (Figure 2). Staging for distant metastases was negative and no other osteochondromas were found. No familial history of osteochondromatosis was referred.
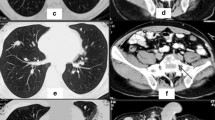
Contrast-enhanced CT scan performed at the time of diagnosis in December 2009. (a) Presence of a large mass arising from an osteochondroma (arrow) of the left iliac bone (coronal plane, bone window, venous phase); (b) the primary tumour appears as a poli-lobulated mass extending within the pelvis, characterised by an irregular, peripheral contrast enhancement and scattered calcification islets (circle) (axial plane, abdomen window, arterial phase).
Tru-cut biopsy of the pelvic, primary tumour, performed in December 2009. Histopathological examination (HE x5, inset x10): fibrous tissue with nests of cartilaginous proliferation with hypercellularity and variation in cellular size and shape, in a focally myxoid matrix. Final diagnosis was G2 peripheral conventional chondrosarcoma. Radiologic features were not consistent with the presence of dedifferentiated areas thus supporting the final diagnosis of a conventional chondrosarcoma.
Front-line surgery was ruled out because of the extent of the disease, the major blood vessels and nerves being involved. In February 2010, chemotherapy with full-dose doxorubicin plus ifosfamide was administered for 3 cycles, but tumour progression ensued. In April 2010, definitive external beam radiotherapy (total dose 72 Gy) achieved a minor dimensional response and symptom control.
In July 2012, the disease progressed locally and gave a single liver metastasis, confirmed on biopsy (Figure 3). Chemotherapy with 14-day prolonged infusion of high-dose ifosfamide was administered for one cycle but had to be withdrawn due to neurotoxicity. Chemotherapy with cisplatin and etoposide for 2 cycles was given, with progression of the disease.
In December 2012, in the lack of alternative options, a fourth-line chemotherapy was started with gemcitabine (1,000 mg/sqm on day 1,8,15, every 28 days, administered intravenously in 30’). By RECIST the disease looked stable with regard to the pelvic, primary lesion, while a partial response of the liver lesion was observed (Figure 4). A significant improvement of symptoms, i.e. pain and walking impairment due to compression of the left femoral nerve by the primary tumour, was achieved, and progressively led to withdraw analgesics, i.e., fentanyl TTS 50 mcg/h and pregabalin 300 mg twice/day.
Contrast-enhanced CT scan of the liver and the pelvis (axial plane, abdomen window, venous phase). The progressive hepatic metastasis (black arrow) before (a) and after (b) chemotherapy with cisplatin/etoposide, then a RECIST response after 4 (c) and 9 (d) cycles of gemcitabine. Pelvis reports (e-h) appear stable (white arrow: primary tumour; asterisk: urinary bladder).
Neutropenia G1-G2 was recorded as the only side effect to gemcitabine.
In September 2013, chemotherapy was interrupted after 9 cycles as for patient request. Tumour response was maintained until February 2014 when, after a-14 month progression-free survival, a disease progression was observed to the liver and to the abdomen, with the new appearance of multiple peritoneal metastases. In May 2014, gemcitabine was restarted, with progression at the following evaluation in September 2014.
Discussion
This is the first report of a response to gemcitabine as single agent, in a patient with a heavily pre-treated advanced cCS. The response was marked by a regression of the tumour and symptomatic improvement. While high-grade chondrosarcoma subtypes (mesenchymal and dedifferentiated) can be sensitive to chemotherapy [9], cCS is usually described as a chemo-refractory disease [10]. Indeed, in high-grade chondrosarcomas responses to chemotherapy have been reported to anthracycline-based chemotherapy combinations, and they are usually treated with regimens commonly used in high-grade osteosarcomas, also including cisplatin [7,11-13].
Trials with targeted-therapy have not proven efficacy so far. The initial enthusiasm on hedgehog inhibitors coming from pre-clinical studies, on the basis of a strong activation of the hedgehog pathway in chondrosarcomas, has been cut down after the results of a phase 2 trial in which no objective responses were observed [14].
Gemcitabine is a nucleoside analogue of deoxycytidine, which metabolizes in cell to its active metabolites gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP) [15]. Gemcitabine, both as single agent or in combination with taxanes, is known to be active in soft tissue sarcomas, especially leiomyosarcoma and angiosarcoma [16-19]. As regards bone sarcomas, some small studies reported objective responses with gemcitabine and docetaxel in osteosarcoma and Ewing’s sarcoma [8,20,21]. Some responses have been reported with gemcitabine-based regimens in the treatment of chondrosarcoma, yet the experience with this combination of drugs is very limited (data summarised in Table 1).
Though a single case, this is the first report of responsiveness of cCS to gemcitabine alone. This patient was experiencing a progressive disease with worsening symptoms before gemcitabine. After starting gemcitabine, pain and walking impairment quickly improved. Tumour response was a RECIST partial response to the site of liver metastasis, and a minor tumour shrinkage of the primary, pretreated with RT, lesion. Response was long lasting. Interestingly, this patient had not responded to any of the drugs used previously.
To be noted, the patient described in this case report carried a secondary peripheral chondrosarcoma. Differently from central variant, secondary peripheral chondrosarcomas have distinct molecular features. In fact, solitary osteochondromas are characterised by homozygous deletion of EXT-1 (exostosin) gene, which is involved in heparan sulfate biosynthesis. This alteration may lead to a dysregulation of downstream signalling pathways and, eventually, progression to secondary peripheral chondrosarcoma [22,23]. On this basis, different targets as compared to primary central chondrosarcoma have been identified [24] in secondary peripheral chondrosarcoma and a different chemosensitivity cannot be excluded. Even if, at least of our knowledge, there are no data to sustain at present a higher sensitivity to gemcitabine in presence of an EXT-1 deletion and the available reports on the activity of gemcitabine/docetaxel in chondrosarcoma do not go into details on the chondrosarcoma subtype, this cannot be ruled out.
Conclusion
We add to anecdotal evidence that gemcitabine-based chemotherapy may be active in cCS. This needs to be confirmed through prospective studies targeted to cCS, without the confounding factor of other histotypes. Whether the combination of docetaxel and gemcitabine can be more active than gemcitabine single agent in cCS is left to understand, considering the extra-toxicity implied by the combination in comparison to single-agent gemcitabine.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: IARC; 2013.
Björnsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–19.
Angelini A, Guerra G, Mavrogenis AF, Pala E, Picci P, Ruggieri P. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929–37.
Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818–31.
Uhl M, Mattke M, Welzel T, Oelmann J, Habl G, Jensen AD, et al. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: first report of long-term results. Cancer. 2014;120:1579–85.
The ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Supplement 3):113–23.
Italiano A, Mir O, Cioffi A, Palmerini E, Piperno-Neumann S, Perrin C, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol. 2013;24:2916–22.
Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, et al. Phase II study of sequential gemcitabine followed by docetaxel for recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally recurrent chondrosarcoma: results of Sarcoma Alliance for Research Through Collaboration Study 003. Oncologist. 2012;17:321.
Frezza AM, Cesari M, Baumhoer D, Biau D, Bielack S, Campanacci DA, et al. Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer. 2015;51:374–81.
Van Maldegem AM, Bovée JV, Gelderblom H. Comprehensive analysis of published studies involving systemic treatment for chondrosarcoma of bone between 2000 and 2013. Clin Sarcoma Res. 2014;4:11.
La Rocca RV, Morgan KW, Paris K, Baeker TR. Recurrent chondrosarcoma of the cranial base: a durable response to ifosfamide-doxorubicin chemotherapy. J Neurooncol. 1999;41:281–3.
Nooij MA, Whelan J, Bramwell VHC, Taminiau AT, Cannon S, Hogendoorn PCW, et al. Doxorubicin and cisplatin chemotherapy in high-grade spindle cell sarcomas of the bone, other than osteosarcoma or malignant fibrous histiocytoma: a European Osteosarcoma Intergroup Study. Eur J Cancer. 2005;41:225–30.
Van Maldegem AM, Gelderblom H, Palmerini E, Dijkstra SD, Gambarotti M, Ruggieri P, et al. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer. 2014;120:3159–64.
Italiano A, Le Cesne A, Bellera C, Piperno-Neumann S, Duffaud F, Penel N, et al. GDC-0449 in patients with advanced chondrosarcomas: a French Sarcoma Group/US and French National Cancer Institute Single-Arm Phase II Collaborative Study. Ann Oncol. 2013;24:2922–6.
Lund B, Kristjansen PE, Hansen HH. Clinical and preclinical activity of 2’,2'-difluorodeoxycytidine (gemcitabine). Cancer Treat Rev. 1993;19:45–55.
Stacchiotti S, Palassini E, Sanfilippo R, Vincenzi B, Arena MG, Bochicchio AM, et al. Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the Italian Rare Cancer Network. Ann Oncol. 2012;23:501–8.
Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol. 2007;25:2755–63.
Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824–31.
Pautier P, Floquet A, Penel N, Piperno-Neumann S, Isambert N, Rey A, et al. Randomized multicenter and stratified phase II study of gemcitabine alone versus gemcitabine and docetaxel in patients with metastatic or relapsed leiomyosarcomas: a Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) French Sarcoma Group. Oncologist. 2012;17:1213–20.
Lee EM, Rha SY, Lee J, Park KH, Ahn J-H. Phase II study of weekly docetaxel and fixed dose rate gemcitabine in patients with previously treated advanced soft tissue and bone sarcoma. Cancer Chemother Pharmacol. 2012;69:635–42.
Navid F, Willert JR, McCarville MB, Furman W, Watkins A, Roberts W, et al. Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer. 2008;113:419–25.
Bovée JVMG, Hogendoorn PCW, Wunder JS, Alman B. Cartilage tumours and bone development: molecular pathology and possible therapeutic targets. Nat Rev Cancer. 2010;10:481–8.
De Andrea CE, Reijnders CMA, Kroon HM, de Jong D, Hogendoorn PCW, Szuhai K, et al. Secondary peripheral chondrosarcoma evolving from osteochondroma as a result of outgrowth of cells with functional EXT. Oncogene. 2012;31(9):1095–104.
Gelderblom H, Hogendoorn PCW, Dijkstra SD, van Rijswijk CS, Krol a. D, Taminiau a. HM, Bovee JVMG. The clinical approach towards chondrosarcoma. Oncologist. 2008;13:320–9.
Merimsky O, Meller I, Flusser G, Kollender Y, Issakov J, Weil-Ben-Arush M, et al. Gemcitabine in soft tissue or bone sarcoma resistant to standard chemotherapy: a phase II study. Cancer Chemother Pharmacol. 2000;45:177–81.
Acknowledgments
The authors thank the patient and her family. We also thank Mireya Fernandez-Fournier for English revision.
Disclosures
NH has received a research grant from the Spanish Society of Medical Oncology SEOM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
SP, NH, PGC and SS received research funding from Lilly in the past 3 years.
Authors’ contributions
SP and NH compiled the clinical data, reviewed the literature and drafted the manuscript. CM provided radiological data. PC provided the pathologic data. MG compiled the clinical data, reviewed and edited the manuscript. PGC offered conceptual advice, reviewed and edited the manuscript. SS compiled the clinical data, offered conceptual advice, guided the composition process, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Provenzano, S., Hindi, N., Morosi, C. et al. Response of conventional chondrosarcoma to gemcitabine alone: a case report. Clin Sarcoma Res 5, 9 (2015). https://doi.org/10.1186/s13569-015-0025-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13569-015-0025-z