Abstract
Background
Spiny-footed lizards constitute a diverse but scarcely studied genus. Microsatellite markers would help increasing the knowledge about species boundaries, patterns of genetic diversity and structure, and gene flow dynamics. We developed a set of 22 polymorphic microsatellite loci for cross-species amplification in three taxa belonging to the Acanthodactylus scutellatus species group, A. aureus, A. dumerili/A. senegalensis and A. longipes, and tested the same markers in two other members of the group, A. scutellatus and A. taghitensis.
Results
Amplifications in A. aureus, A. longipes and A. dumerili/A. senegalensis were successful, with markers exhibiting a number of alleles varying between 1 and 19. Expected and observed heterozygosity ranged, respectively, between 0.046–0.893 and 0.048–1.000. Moreover, 17 and 16 loci were successfully amplified in A. scutellatus and A. taghitensis, respectively.
Conclusion
These markers are provided as reliable genetic tools to use in future evolutionary, behavioural and conservation studies involving species from the A. scutellatus group.
Similar content being viewed by others
Background
Spiny-footed lizards, or fringe-toed lizards (genus Acanthodactylus), form a clade of small ground-dwelling lizards occurring mostly in arid regions [1, 2]. The genus is the most specious of the Lacertidae family and is widely distributed, occurring from the Iberian Peninsula, south of the Mediterranean Basin, across the Sahara-Sahel, Arabian Peninsula, and as far east as India [1, 2]. Being often abundant and occupying different types of open, flat habitats, these lizards are important elements of the vertebrate communities of deserts and arid ecosystems in North Africa and Arabia. Despite their diversity, knowledge about most of the species is still scarce and their taxonomy is partly unresolved [1–4]. Most authors agree on splitting Acanthodactylus into several species groups or complexes [1, 4]. The A. scutellatus species group shows one of most complex taxonomies [2, 5–8]. It includes six species according to the last global revision (A. aureus, A. dumerili, A. longipes, A. scutellatus, A. senegalensis and A. taghitensis) [3]. However, urgent systematic revision based on molecular data is needed given that: (1) eastern populations previously attributed to A. longipes are now considered a new species (A. aegyptius, [7]); and (2) species boundaries in A. scutellatus, A. longipes, A. dumerili and A. senegalensis as currently defined remain uncertain (own unpublished data, SC Lopes, Velo-Antón, Crochet, Brito). The species group has multiple forms occurring in sympatry in Mauritania—A. aureus, A. dumerili, A. senegalensis, and A. longipes [9]. In this contact zone, morphologically intermediate individuals were previously observed [3] and molecular studies are needed to distinguish whether high morphological diversity or hybridization explain these intermediate morphotypes. In addition, assessment of gene flow in such areas of sympatry would be critical for a better understanding of the species boundaries. Microsatellite markers have been extremely useful, and affordable, for addressing numerous topics in conservation and evolutionary biology, allowing, e.g., gene flow and population structure assessments, demographic inferences and genetic diversity estimation [10–12]. Yet, no microsatellite markers are available for the Acanthodactylus genus.
Here we describe a set of 22 polymorphic microsatellite loci (tri- and tetranucleotides) characterized in four species included in the A. scutellatus species group (A. aureus, A. longipes and A. dumerili/A. senegalensis). Considering the uncertain species boundaries for A. dumerili and A. senegalensis, we chose to refer to them as A. dumerili/A. senegalensis in the following sections. We further tested cross-amplification of these markers in two other members of the species group, A. scutellatus and A. taghitensis.
Methods
A genomic library was constructed from 12 specimens of A. aureus, collected across the species’ distribution. A tissue sample was collected from the tail tip by following ethical guidelines for use of live reptiles (http://www.aaalac.org/accreditation/Guidelines_for_Use_of_Live_Amphibians_and_Reptiles.pdf). All specimens were released on site after sample collection. Fieldwork was developed with permission from the Ministére Délégué auprès du Premier Ministre Chargé de l’Environnement, Nouakchott (Permit: 460/MDE/PNBA) and from the Haut Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification, Rabat (Permits 256-2012 and 20-2013). Analyses were done at a CITES registered laboratory: 13PT0065/S. Field collection and handling practices were approved by the Committee of Animal Experimentation of the University of Porto (Portugal) under the Directive 2010/63/EU of the European Parliament.
Genomic DNA extractions were performed from tissue samples using EasySpin Kit (Qiagen), following an adapted protocol for tissue samples (with minor adjustments to centrifugation and incubation conditions) and then pooled in equimolar concentrations. The changes to the extraction protocol were as follows: after adding the AB solution, we centrifuged at 3700 rpm for 4 min (instead of 4000 rpm for 2 min). After adding the Wash solution, we centrifuged at 3700 rpm for 6 min (instead of 8000 rpm for 1 min). After repeating the Wash solution step and discarding flow-through, we centrifuged at 3700 rpm for 10 min (instead of 14,000 rpm for 5 min). After adding the Elution Buffer, we incubated at 55° for 15 min (instead of 50° for 10 min). Last centrifugation was at 3700 rpm (instead of 14,000 rpm). Microsatellite isolation was developed through 454 GS-FLX Titanium pyrosequencing of enriched DNA libraries [13]. This process was developed by GenoScreen (http://www.pasteur-lille.fr/fr/recherche/plateformes/tordeux_plat.html) and included sequence data quality control, assembly and analyses, and primer design. Initially, 50 loci were selected from the library and tested for amplification using seven samples of A. aureus, A. dumerili/A. senegalensis, and A. longipes. Thirty loci amplified reliably, producing fragments of the expected size. Twenty-two were polymorphic (Table 1), and amplified with differential success in the following target species: 21 in A. aureus, 18 in A. longipes and 15 in A. dumerili/A. senegalensis. These 22 loci were therefore used for genotyping 38 samples of A. aureus, 35 of A. longipes, and 43 of A. dumerili/A. senegalensis, collected along coastal Morocco and Mauritania (Table 2; Fig. 1). Markers were multiplexed in four reactions, using M13-primer genotyping protocol with four different dye-labelled tails, and forward primer concentration of 1/10 of dye-labelled reverse primer [14] (Table 1). The transferability of the primers was tested by cross-amplification of five specimens of A. scutellatus (from Morocco, Tunisia, Libya, Algeria and Egypt) and one specimen of A. taghitensis (Mauritania). PCR amplifications were conducted using the Multiplex PCR Kit (QIAGEN) following manufacturer’s instructions in a final 10 μl volume, always in the presence of a negative control. Touchdown PCR conditions started with an initial denaturation step of 15 min at 95 °C; first round (nine cycles) of 30 s at 95 °C, 90 s for annealing (decreasing 0.5 °C per cycle) at 58–54 °C (Multiplexes 1, 2 and 3) or 55–51 °C (Multiplex 4), and 30 s at 72 °C; second round (31 cycles) of 30 s at 95 °C, 1 min at 54 °C (Multiplexes 1, 2 and 3), or 51 °C (Multiplex 4), 30 s at 72 °C, and a final extension of 30 min at 60 °C. Amplification was performed in Biorad T100 Thermal Cyclers, and the PCR products were later separated by capillary electrophoresis on an automatic sequencer ABI3130xl Genetic Analyzer (AB Applied Biosystems). Fragments were scored against the GeneScan-500 LIZ Size Standard using the GENEMAPPER 4.1 (Applied Biosystems) and manually checked twice. Potential evidences of null alleles, allelic dropouts and stuttering were assessed using MICRO-CHECKER v2.2.3 [15] at each locus, for each population. Tests for Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium (LD) were assessed in GENEPOP online version (http://wbiomed.curtin.edu.au/genepop/); with subsequent Bonferroni correction in both cases. Observed and expected heterozygosity were computed using GenAlEx v6.501 [16]. For some populations, samples were obtained from different localities. Consequently, analyses were based on groups of samples that are not necessarily panmitic populations, which probably accounts for deviations from Hardy–Weinberg equilibrium.
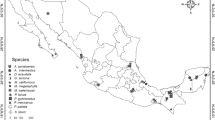
Distribution of genotyped samples used for Acanthodactylus aureus, A. dumerili/senegalensis and A. longipes. White circles correspond to Pop1, while grey circles correspond to Pop2. Circles are proportional to sample size. The rectangle in the map of A. aureus represents the area depicted in the maps of A. dumerili/senegalensis and A. longipes. The samples sizes of the populations are the following: Pop1 = 21 and Pop2 = 17 in A. aureus; Pop1 = 24 and Pop2 = 19 in A. dumerili/A. senegalensis; and Pop1 = 14 and Pop2 = 19 in A. longipes
Results and discussion
MICRO-CHECKER revealed no evidence of allelic dropout or stuttering, and no heterozygote excess was observed. In addition, no loci appeared to be in linkage disequilibrium. Table 3 summarizes occurrence of heterozygote deficiency and suspected null alleles for all loci in all populations in the three target species. While the occurrence of null alleles would limit the use of some of these markers in the affected species, other departures from Hardy–Weinberg equilibrium probably result from pooling several sampling localities in the same “populations” (see above). Additionally, even markers showing such evidences might be adequate to apply in other populations and they are applicable in at least one of these species.
All loci genotyped for each species were polymorphic (Table 4), except for Ac44 that amplified only for A. longipes. The Ac36 was also monomorphic in A. dumerili/A. senegalensis tested populations but polymorphism was observed in inland samples of this species (own unpublished data, Lopes, Velo-Antón, Crochet, Brito). The number of alleles per locus varied between 5 and 19 in A. aureus, and between 1 and 9 in A. dumerili/A. senegalensis and A. longipes. Expected and observed heterozygosity varied, respectively, between 0.594–0.893/0.188–1.000 in A. aureus, 0.223–0.829/0.154–0.826 in A. dumerili/A. senegalensis (ignoring Ac36), and 0.046–0.862/0.048–0.905 in A. longipes (ignoring Ac44). Most markers amplified in both A. scutellatus/17 loci) and A. taghitensis (16 loci).
Although the applicability of each marker may depend on the species considered, the information provided in our work allows a selection of good markers for future use on assessments of genetic structure, genetic diversity, gene flow, and demographic inferences, expanding the possible themes for evolutionary, behavioural and conservation studies in this species group.
References
Salvador A. A revision of the lizards of the genus Acanthodactylus (Sauria: Lacertidae). Bon Zool Monog. 1982;16:1–167.
Arnold EN. Osteology, genitalia and the relationships of Acanthodactylus (Reptilia: Lacertidae). Bull Brit Mus Nat Hist (Zoology). 1983;44:291–339.
Crochet PA, Geniez P, Ineich I. A multivariate analysis of the fringe-toed lizards of the Acanthodactylus scutellatus group (Squamata: Lacertidae): systematic and biogeographical implications. Zool J Linnean Soc. 2003;137:117–55.
Harris D, Arnold E. Elucidation of the relationships of spiny-footed lizards, Acanthodactylus spp. (Reptilia: Lacertidae) using mitochondrial DNA sequence, with comments on their biogeography and evolution. J Zool. 2000;252:351–62.
Bons J. Les lacertiliens du Sud-Ouest Marocain: systématique, répartition géographique, éthologie, écologie). Trav Inst Sci Chérifien. 1959;18:1–130.
Mellado J, Olmedo G. El género Acanthodactylus en Marruecos: problemas de identificación en los grupos de especies A. pardalis y A. scutellatus. Amphib Reptilia. 1990;11:131–46.
Baha El Din SM. A contribution to the herpetology of Sinai. Brit Herp Soc Bul. 1994;48:18–27.
Baha El Din SM. A new lizard of the Acanthodactylus scutellatus group (Squamata: Lacertidae) from Egypt. Zool Middle East. 2007;40:21–32.
Sindaco R, Jeremcenko VK. The reptiles of the Western Palearctic: annotated checklist and distributional atlas of the turtles, crocodiles, amphisbaenians and lizards of Europe, North Africa, Middle East and Central Asia. Latina: Edizioni Belvedere, Monografie della Societas Herpetologica Italica; 2008.
Schlotterer C. The evolution of molecular markers: just a matter of fashion? Nat Rev Genet. 2004;5:63–9.
Wan QH, Wu H, Fujihara T, Fang SG. Which genetic marker for which conservation genetics issue? Electrophoresis. 2004;25:2165–76.
Selkoe KA, Toonen RJ. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett. 2006;9:615–29.
Malausa T, Gilles A, Meglecz E, Blanquart H, Duthoy S, Costedoat C, et al. High-throughput microsatellite isolation through 454 GS-FLX titanium pyrosequencing of enriched DNA libraries. Mol Ecol Res. 2011;11:638–44.
Schuelke M. An economic method for the fluorescent labelling of PCR fragments. Nat Biotech. 2000;18:233–4.
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P. MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes. 2004;4:535–8.
Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics. 2012;28:2537–9.
Authors’ contributions
SCL carried out the laboratory tasks, performed the molecular analyses, and drafted the manuscript. PP and SL participated in the microsatellite marker optimization and validation. GVA, PAC and RG contributed to the molecular analyses. JCB designed and supervised the study. All authors read and approved the final manuscript.
Acknowledgements
This study was partially supported by Mohammed bin Zayed Species Conservation Fund (project 11052709), by National Geographic Society (Grants CRE 7629-04 and 8412-08), by FEDER funds through the Operational Programme for Competitiveness Factors—COMPETE (FCOMP-01-0124-FEDER-028276), and by National Funds through FCT, Foundation for Science and Technology (PTDC/BIA-BIC/2903/2012). Research conducted in the scope of the LIA known as “Biodiversity and Evolution”. GVA, RG and JCB are supported by FCT contracts (IF/01425/2014, IF/00564/2012, and IF/00459/2013, respectively).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lopes, S.C., Velo-Antón, G., Pereira, P. et al. Development and characterization of polymorphic microsatellite loci for spiny-footed lizards, Acanthodactylus scutellatus group (Reptilia, Lacertidae) from arid regions. BMC Res Notes 8, 794 (2015). https://doi.org/10.1186/s13104-015-1779-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1779-3