Abstract
Background
Copy Number Variants (CNVs) is a new molecular frontier in clinical genetics. CNVs in 1p36 are usually pathogenic and have attracted the attention of cytogeneticists worldwide. None of 1p36 triplication has been reported thus far.
Results
We present three patients with CNVs in 1p36. Among them one is the first 1p36 tetrasomy due to a pure microtriplication and the other two are 1p36 microdeletion. Traditional chromosome G-banding technique showed a normal karyotype. Single nucleotide polymorphism (SNP) microarray analysis combined with multiplex ligation-dependent probe amplification (MLPA) and fluorescence in situ hybridization (FISH) were used to identify and confirm the chromosome microdeletion/microtriplication. The facial dysmorphisms of the patient with 1p36 tetrasomy differed from those two patients with 1p36 monosomy. The expression levels of B3GALT6, MIB2, PEX10 and PANK4 in the blood were determined, and differential expressions were observed between the patients and controls.
Conclusions
Our study shows the first case of 1p36 tetrasomy due to a pure microtriplication in a patient with severe intellectual disability and seizures. The study provides a new resource for studying the mechanisms of microtriplication formation, and provides an evidence that overexpression of the specific genes might be related the specific phenotype of 1p36 microtriplication.
Similar content being viewed by others
Background
Copy Number Variants (CNVs) is a new molecular frontier in clinical genetics [1]. Some CNVs are associated with genomic disorders with extreme phenotypic heterogeneity, which brings challenges in genetic diagnosis, counseling, and management [2]. CNVs in 1p36 include microdeletion and microduplication. 1p36 microdeletion is relatively common, with an incidence of ~1 in 5000 newborns [3],[4]. Its main phenotypes include intellectual disability, developmental delay, prominent forehead, low-set ears, long philtrum, pointed chin, short feet, hearing loss, seizures, hypomyotonia, feeding difficulties, speech delay, strabismus, heart defects, obesity, skeletal anomalies, renal and genital abnormalities [3],[4].
In contrast, 1p36 microduplication is rare, with only two cases of "pure" 1p36 microduplication having been reported, one affecting a 6-month-old male infant and the other, a 17-year-old male patient [5]. The major phenotypic features in these two patients were hypotonia, severe psychomotor delay, intellectual disability, developmental delay and speech defects.
Here, we present three patients with CNVs in 1p36. Among them one is the first 1p36 tetrasomy due to a pure microtriplication and the other two are 1p36 microdeletion. Changes in chromosome copy numbers were detected and confirmed by SNP array, MLPA and FISH analyses. Expression levels of 4 genes (B3GALT6, MIB2, PEX10 and PANK4) mapping to the deleted and triplicated segments were determined by quantitative PCR analysis, which showed that transcript levels correlated with altered DNA copy numbers, indicating that the observed phenotypes resulted from gene-dosage effects.
Case presentation
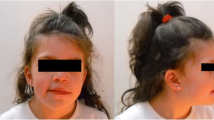
An eight-year-old female patient was brought to our clinic by her non-sanguineous parents due to severe intellectual disability and seizures. The patient, the first and only child of a 24-year-old father and a 22-year-old mother, was born at full term by a normal delivery after an uneventful pregnancy. She had suffered from feeding problems in infancy as well as a medical history of severe seizures. On top of that, she could also catch a cold and fever very easily, and had once been hospitalized because of pneumonia. She developed delay obviously at each developmental milestone, including gross motor, fine motor, language, cognitive development and social behavior. For instance, she was capable of walking until 2 years and half and saying "dada" and "mama" until 2 years. She undergone surgery for congenital left eyelid ptosis at the age of 3 years. Now she was 8 years old, and according to our physical examination, her weight, height and body mass index (BMI) represented were 18 kg, 108.5 cm, and 15.29 kg/m2 (25th 50th centiles) [6], respectively; Also, she has got microcephaly (head circumference, 49.6 cm; reference value, 52.19 cm). Facial dysmorphism, with strabismus, hypertelorism, low hairline, ear malformations, broad nasal bridge, wide mouth, thick lips and prominent incisors could be observed (Figure 1; Table 1). The child could understand some simple sentences, whose expressive ability to speak simple words and sentences is extremely poor. It can be seen that she had behavioral abnormalities, unable to take care of herself, no to mention getting focused and controlling herself well. However, ultrasonography of the uterus, liver, gallbladder, spleen, pancreas, kidneys and heart showed no abnormalities.
Other two 8-year-old female patients with 1p36 microdeletion syndrome were also identified in the study by FISH and MLPA techniques (Additional file 1).
Results
Karyotype analysis at 550 band resolution showed the proband with a female karyotype(46,XX), without any suggestion of chromosome abnormality (Figure 2A). Her parents also presented normal karyotype.
Copy number variants analysis of the patient: (A) "Normal" high-resolution karyotype. (B) A SNP microarray analysis detected an approximately 5.28-Mb triplication in the short arm of chromosome 1. (C) MLPA result using SALSA kit P147-B1 of MRC Holland, showing a triplication included all probes from ISG15 up to AJAP1(RPH are approximately 2.0). (D) FISH results of the patient. Two red signals with the similar strength were presented in the long arm of chromosome 1 by the control probe TelVysion 1q. However, by the testing probe TelVysion 1p, the green hybridization signal in one 1p was obviously stronger than that in the other 1p, and in interphase nucleus, four green signals could be seen. (E) FISH image of the parents of the patient showed normal appearance, indicating that the 1p partial triplication is de novo (the FISH image of mother didn't shown). (F) Quantitative PCR analysis showed the patient had 3 to 6 times as higher expression levels than control for the four genes B3GALT6, MIB2, PEX10, PANK4 in the blood.
Single nucleotide polymorphism (SNP) microarray analysis by Affymetrix SNP 6.0 was performed as our previous report [8],[9]. The result showed a pure 5.28 Mb microtriplication at 1p36.33p36.32, and the karyotype could be expressed as 46,XX.arr[hg19]1p36.33p36.32(1-5,280.000) × 4 (Figure 2B), which was considered as a pathogenic CNV according to the available databases including Database of Genomic Variant (http://projects.tcag.ca/variation/), DECIPHER (https://decipher.sanger.ac.uk/), ECARUCA (http://umcecaruca01.extern.umcn.nl:8080/ecaruca/ecaruca.jsp), OMIM (http://www.omim.org/), and pubmed (http://www.pubmed.gov/).
Multiplex ligation dependent probe amplification (MLPA) analysis using the SALSA kit P147-B1 (MRC-Holland, Amsterdam, the Netherlands, see http://www.mlpa.com) confirmed the microtriplication for chromosome 1p (Figure 2C), including all probes from ISG15 up to AJAP1, which was consistent with the SNP microarray result. The SNP array result was also confirmed by Fluorescent in situ hybridization (FISH) with two subterminal probes TelVysion 1p (SpectrumGreen labeled) and TelVysion 1q (SpectrumOrange labeled) which were purchased from Abbott-Vysis, Inc (Downers Grove, IL, USA) (Figure 2D and 2E).
Quantitative PCR analysis showed that transcript levels in the blood correlate with altered DNA copy numbers. For the selected 4 OMIM genes B3GALT6 (betaGal beta 1,3-galactosyltransferase polypeptide 6), MIB2 (mindbomb E3 ubiquitin protein ligase 2), PEX10 (peroxisomal biogenesis factor 10), PANK4 (pantothenate kinase 4), which are situated in the 1p36.33p36.32 region and were expression in blood, the ratios of the patient to the controls (a healthy 8-year-old female sample) showed significant differences (Figure 2F), indicating upregulated and deregulated expression respectively.
Discussion
Here, we report the first case of a pure 1p36.33p36.32 tetrasomy resulting from a de novo microtriplication detected on SNP microarray and confirmed by MLPA and FISH analyses. Our results provide a new case for exploring the formation mechanism of 1p36 microtriplication and the molecular etiology of the patient.
The most common mechanism underlying CNVs is non-allelic homologous recombination and not non-homologous end joining mediated by low copy repeats during meiosis [10]. Cytogenetically invisible tetrasomy due to a chromosome segment microtriplication is a very complex and rare CNV. Till date, only a few triplications have been reported, including 2q11.2 → q21 [11], 2q12.3 → q13 [12], 3q25.3 → q29 [13], 7q11.23 [14], 13q14 [15], 15q11 → q13 [16], 17p11.2 → p12 [17] and 22q11.2 [18]. A case of "pure" 1p36 tetrasomy has not yet been reported, although complex rearrangements resulting in deletions, duplications and/or triplications for portions of 1p36 have been reported elsewhere, along with postulated mechanisms of formation [19],[20]. Thus, our study provides a new resource for studying the mechanisms of microtriplication formation.
In general, a tetrasomy causes a similar but more severe phenotype than a trisomy of the same region. For example, 7q11.23 duplication causes Williams-Beuren syndrome (WBS). The first patient reported to have a 7q11.23 triplication had a similar but more severe WBS phenotype, with severe developmental delay, severe retardation in language and speech, behavioral problems, autistic features and mild dysmorphic features [14]. Girirajan et al. described a patient with a large 17p11.2p12 duplication, partial 17p11.2p12 triplication and 17q11.2q12 deletion [17]. The severe phenotypic features in the patient included heart defects, seizure, thoracolumbar fistula and malignant hyperthermia. They attributed the disease-causing events to an increased copy number of dosage-sensitive genes in 17p11.2p12. We found that the clinical phenotypes of our patient with microtriplication 1p36 were similar to our other two patients with monosomy 1p36 (Table 1). These phenotypes included intellectual disability, developmental delay, feeding difficulties, hyperactivity and seizures. The two reported cases of 1p36 trisomy [5] had similar phenotypes to those in our patients with 1p36 tetrasomy and 1p36 deletion, but the clinical symptoms (psychomotor delay, intellectual disability, speech defects) were relatively mild.
The reason why 1p36 triplication causes more severe phenotype than 1p36 duplication, the dosage effect of gene expression in this region would be one of the possibilities. For example, craniosynostosis has been reported in patients with deletions and duplications/triplications of a gene on 1p36 that plays a role in regulating cranial suture closure [21]. In this 1p36 microtriplication patient, we found a de novo 5.28 Mb microtriplication at 1p36.33p36.32, involving 152 genes and 49 OMIM morbid genes. We hypothesize that the dosage effect of gene is related with the clinical phenotype of the patient. We selected four OMIM genes B3GALT6, MIB2, PEX10, PANK4 which were expressed in the blood to test the hypothesis, and the results showed the patient had 3 to 6 times as higher expression levels than that in the control, which provided an evidence that overexpression of the specific genes might be related the specific phenotype of 1p36 microtriplication.
Conclusions
Our study shows the first case of 1p36 tetrasomy due to a pure microtriplication in a patient with severe intellectual disability and seizures. The study provides a new resource for studying the mechanisms of microtriplication formation, and provides an evidence that overexpression of the specific genes might be related the specific phenotype of 1p36 microtriplication.
Consent
Written informed consent was obtained from the parents of the patients for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editors-in-Chief of this journal.
Authors' contribution
All authors read and approved the final manuscript. YQT designed the study and revised the manuscript. FX performed the MLPA and quantitative PCR, analyzed the data and wrote the paper. YNZ and CDH performed the FISH experiments and interpreted the results. KT performed the SNP experiments. CGZ reviewed all laboratory results and patient data. GL and GXL conceived the study and provided financial support.
Additional file
References
Moreno-De-Luca D, Cubells JF: Copy number variants: a new molecular frontier in clinical psychiatry. Curr Psychiatry Rep 2011, 13: 129–137. 10.1007/s11920-011-0183-5
Girirajan S, Rosenfeld JA, Coe BP, Parikh S, Friedman N, Goldstein A, Filipink RA, McConnell JS, Angle B, Meschino WS, Nezarati MM, Asamoah A, Jackson KE, Gowans GC, Martin JA, Carmany EP, Stockton DW, Schnur RE, Penney LS, Martin DM, Raskin S, Leppig K, Thiese H, Smith R, Aberg E, Niyazov DM, Escobar LF, El-Khechen D, Johnson KD, Lebel RR, et al.: Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N Engl J Med 2012, 367: 1321–1331. 10.1056/NEJMoa1200395
Gajecka M, Mackay KL, Shaffer LG: Monosomy 1p36 deletion syndrome. Am J Med Genet C: Semin Med Genet 2007, 145C: 346–356. 10.1002/ajmg.c.30154
Battaglia A, Hoyme HE, Dallapiccola B, Zackai E, Hudgins L, McDonald-McGinn D, Bahi-Buisson N, Romano C, Williams CA, Brailey LL, Zuberi SM, Carey JC: Further delineation of deletion 1p36 syndrome in 60 patients: a recognizable phenotype and common cause of developmental delay and mental retardation. Pediatrics 2008, 121: 404–410. 10.1542/peds.2007-0929
Giannikou K, Fryssira H, Oikonomakis V, Syrmou A, Kosma K, Tzetis M, Kitsiou-Tzeli S, Kanavakis E: Further delineation of novel 1p36 rearrangements by array-CGH analysis: narrowing the breakpoints and clarifying the "extended" phenotype. Gene 2012, 506: 360–368. 10.1016/j.gene.2012.06.060
Ma J, Wang Z, Song Y, Hu P, Zhang B: BMI percentile curves for Chinese children aged 7-18 years, in comparison with the WHO and the US Centers for Disease Control and Prevention references. Public Health Nutr 2010, 13: 1990–1996. 10.1017/S1368980010000492
Bursztejn AC, Bronner M, Peudenier S, Grégoire MJ, Jonveaux P, Nemos C: Molecular characterization of a monosomy 1p36 presenting as an Aicardi syndrome phenocopy. Am J Med Genet A 2009, 149A: 2493-2500.
Cheng DH, Gong F, Tan K, Lu CF, Lin G, Lu GX, Tan YQ: Karyotype determination and reproductive guidance for short stature women with a hidden Y chromosome fragment. Reprod Biomed Online 2013, 27: 89–95. 10.1016/j.rbmo.2013.03.015
Tan YQ, Tan K, Zhang SP, Gong F, Cheng DH, Xiong B, Lu CF, Tang XC, Luo KL, Lin G, Lu GX: Single-nucleotide polymorphism microarray-based preimplantation genetic diagnosis is likely to improve the clinical outcome for translocation carriers. Hum Reprod 2013, 28: 2581–2592. 10.1093/humrep/det271
Carvalho CM, Lupski JR: Copy number variation at the breakpoint region of isochromosome 17q. Genome Res 2008, 18: 1724–1832. 10.1101/gr.080697.108
Cooke LB, Richards H, Lunt PW, Burvill-Holmes L, Howell RT, McDermott A: Duplication 2 (q11.2 → q21): a previously unreported abnormality. J Med Genet 1995, 32: 825–826. 10.1136/jmg.32.10.825
Mercer CL, Browne CE, Barber JC, Maloney VK, Huang S, Thomas NS, Foulds N, MacLachlan N: A complex medical phenotype in a patient with triplication of 2q12.3 to 2q13 characterized with oligonucleotide array CGH. Cytogenet Genome Res 2009, 124: 179–186. 10.1159/000207526
Ounap K, Ilus T, Bartsch O: A girl with inverted triplication of chromosome 3q25.3/q29 and multiple congenital anomalies consistent with 3q duplication syndrome. Am J Med Genet A 2005, 134: 434–438. 10.1002/ajmg.a.30134
Beunders G, van de Kamp JM, Veenhoven RH, van Hagen JM, Nieuwint AW, Sistermans EA: A triplication of the Williams-Beuren syndrome region in a patient with mental retardation, a severe expressive language delay, behavioural problems and dysmorphisms. J Med Genet 2010, 47: 271–275. 10.1136/jmg.2009.070490
Brecevic L, Basaran S, Dutly F, Röthlisberger B, Schinzel A: Tandem triplication of chromosome 13q14 with inverted interstitial segment in a 4 year old girl. J Med Genet 2000, 37: 964–967. 10.1136/jmg.37.12.964
Roberts SE, Dennis NR, Browne CE, Willatt L, Woods G, Cross I, Jacobs PA, Thomas S: Characterisation of interstitial duplications and triplications of chromosome 15q11-q13. Hum Genet 2002, 110: 227–234. 10.1007/s00439-002-0678-6
Girirajan S, Williams SR, Garbern JY, Nowak N, Hatchwell E, Elsea SH: 17p11.2p12 triplication and del(17)q11.2q12 in a severely affected child with dup(17)p11.2p12 syndrome. Clin Genet 2007, 72: 47–58. 10.1111/j.1399-0004.2007.00831.x
Yobb TM, Somerville MJ, Willatt L, Firth HV, Harrison K, MacKenzie J, Gallo N, Morrow BE, Shaffer LG, Babcock M, Chernos J, Bernier F, Sprysak K, Christiansen J, Haase S, Elyas B, Lilley M, Bamforth S, McDermid HE: Microduplication and triplication of 22q11.2 a highly variable syndrome. Am J Hum Genet 2005, 76: 865–876. 10.1086/429841
Gajecka M, Yu W, Ballif BC, Glotzbach CD, Bailey KA, Shaw CA, Kashork CD, Heilstedt HA, Ansel DA, Theisen A, Rice R, Rice DP, Shaffer LG: Delineation of mechanisms and regions of dosage imbalance in complex rearrangements of 1p36 leads to a putative gene for regulation of cranial suture closure. Eur J Hum Genet 2005, 13: 139–149. 10.1038/sj.ejhg.5201302
D'Angelo CS, Gajecka M, Kim CA, Gentles AJ, Glotzbach CD, Shaffer LG, Koiffmann CP: Further delineation of nonhomologous-based recombination and evidence for subtelomeric segmental duplications in 1p36 rearrangements. Hum Genet 2009, 125: 551–563. 10.1007/s00439-009-0650-9
D'Angelo CS, Kohl I, Varela MC, de Castro CI, Kim CA, Bertola DR, Lourenço CM, Koiffmann CP: Extending the phenotype of monosomy 1p36 syndrome and mapping of a critical region for obesity and hyperphagia. Am J Med Genet A 2010, 152A: 102–110. 10.1002/ajmg.a.33160
Acknowledgements
We are grateful to the family who participated in this study. This work was supported by the grants from the Major State Basic Research Development Program of China (No. 2012CB944901) and National Natural Science Foundation of China (No. 81471432).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Xu, F., Zhang, YN., Cheng, DH. et al. The first patient with a pure 1p36 microtriplication associated with severe clinical phenotypes. Mol Cytogenet 7, 64 (2014). https://doi.org/10.1186/s13039-014-0064-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13039-014-0064-9