Abstract
Background
Multiple studies regarding the use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in patients with non-compressible torso injuries and uncontrolled haemorrhagic shock were recently published. To date, the clinical evidence of the efficacy of REBOA is still debated. We aimed to conduct a systematic review assessing the clinical efficacy and safety of REBOA in patients with major trauma and uncontrolled haemorrhagic shock.
Methods
We systematically searched MEDLINE (PubMed), EMBASE and CENTRAL up to June 2020. All randomized controlled trials and observational studies that investigated the use of REBOA compared to resuscitative thoracotomy (RT) with/without REBOA or no-REBOA were eligible.
We followed the PRISMA and MOOSE guidelines. Two authors independently extracted data and appraised the risk of bias of included studies. Effect sizes were pooled in a meta-analysis using random-effects models. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation methodology. Primary outcomes were mortality, volume of infused blood components, health-related quality of life, time to haemorrhage control and any adverse effects. Secondary outcomes were improvement in haemodynamic status and failure/success of REBOA technique.
Results
We included 11 studies (5866 participants) ranging from fair to good quality. REBOA was associated with lower mortality when compared to RT (aOR 0.38; 95% CI 0.20–0.74), whereas no difference was observed when REBOA was compared to no-REBOA (aOR 1.40; 95% CI 0.79–2.46). No significant difference in health-related quality of life between REBOA and RT (p = 0.766). The most commonly reported complications were amputation, haematoma and pseudoaneurysm. Sparse data and heterogeneity of reporting for all other outcomes prevented any estimate.
Conclusions
Our findings on overall mortality suggest a positive effect of REBOA among non-compressible torso injuries when compared to RT but no differences compared to no-REBOA. Variability in indications and patient characteristics prevents any conclusion deserving further investigation. REBOA should be promoted in specific training programs in an experimental setting in order to test its effectiveness and a randomized trial should be planned.
Similar content being viewed by others
Background
Haemorrhage from non-compressible torso injuries is a leading cause of death in military and civilian trauma [1]. To control exsanguinating bleeding from non-compressible torso injuries, a damage control approach should be used. A variety of damage control surgery techniques have been developed to limit blood loss, control contamination and preserve one’s physiology such as abdominal packing, non-essential organ removal, extra-peritoneal packing, stapler resection of the bowel, vascular shunts and interventional radiology with embolization procedures.
Resuscitative thoracotomy is commonly used in patients in extremis or cardiac arrest for open cardiac massage and aortic cross-clamping [2, 3]. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is a technique for temporary cessation or limitation of blood flow through the aorta, which may be used as a bridge until definitive control of the bleeding by endovascular procedures or surgery is performed [4]. After emergency room extended focused assessment sonography for trauma (E-FAST) and chest and pelvis x-ray, the balloon can be inflated in the descending thoracic aorta (zone 1) to reduce blood flow below the diaphragm or in the abdominal aorta below the renal arteries (zone 3) to stop bleeding from the pelvis and lower extremities.
The application of a REBOA has been suggested in the following cases: (i) in zone 1 for imminent traumatic cardiac arrest for probable haemorrhagic cause [5]; (ii) in zone 1 for severe haemorrhagic shock due to abdominal and/or pelvic injuries [6, 7]; (iii) in zone 3 for severe pelvic fracture [7, 8] or to control junctional bleeding from the groin end lower extremities; (iv) in zone 1 for penetrating thoracic trauma, according to an algorithm proposed in 2020 [9].
In recent years, REBOA has received a lot of attention for its applicability and promise in adult major trauma settings. It is a less invasive method of haemodynamic control in severe haemorrhagic settings relative to other damage control techniques. Survival benefits between REBOA and non-REBOA in severe abdominal-pelvic haemorrhage and between REBOA and resuscitative thoracotomy (RT) in imminent cardiac arrest for haemorrhage are controversial. All procedures may lead to unintended adverse effects [10] and this underlines the need for shared indications for the use.
The aim of this systematic review was to explore to the best of current knowledge if REBOA is clinically safe and effective in the management of major exsanguination from torso injuries due to trauma.
Methods
We conducted a systematic review to support the major trauma integrated management guideline panel of the Italian National Institute of Health (Istituto Superiore di Sanità) in formulating recommendations [11]. Following the GRADE-ADOPOLMENT methodology [12] and in accordance with the standards defined by the Sistema Nazionale Linee Guida (SNLG) [13], the multidisciplinary panel decided to develop a “de novo” question addressing the efficacy of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) on patients with major trauma. The clinical question addressed in this systematic review was: Is Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) clinically effective in the Management of major exsanguination in trauma?
Registered protocol
The protocol of the present systematic review is stored at the following link: https://osf.io/ntxvj/. We conducted the systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guideline [14, 15].
Inclusion criteria
Randomized controlled trials (RCT) and/or observational studies were included if they met the following criteria: (1) population: children, young people and adults experiencing major trauma, blunt or penetrating; (2) intervention: REBOA; (3) comparison: RT (with/without REBOA) or no REBOA intervention; (4) setting: pre-hospital, emergency department and operating room resuscitation phase. Studies including patients with trauma resulting from burns were excluded.
Outcome measures and follow-up assessment
Primary outcome measures selected for the analyses were as follows: (i) 24-h mortality, 30 days to 12 months mortality; (ii) volume of infused blood components; (iii) health-related quality of life (e.g. Discharge Glasgow Coma Scale); (iv) adverse effects (e.g. amputation); (v) time to haemorrhage control. Secondary outcomes were as follows: (vi) improvement in haemodynamic status and (vii) failure/Success of REBOA technique.
Search strategy
Two professional librarians interviewed the following electronic databases: MEDLINE (PubMed), EMBASE (Elsevier, EMBASE.com) and CENTRAL up to June 9, 2020, with language restricted to English, Italian, Spanish, French, German using the search strategy outlined in Supplement A. We checked the reference lists of all studies included and of any systematic reviews we identified during the search process (including grey literature and conference proceedings). We also searched for ongoing trials (i.e. clinical trials.gov).
Study selection and data extraction
Two independent authors (SG, GC) screened titles and abstracts by the search strategy. Following the first phase, they independently assessed the full text of potentially relevant studies for inclusion. Any disagreement was solved by a discussion with one of the authors (OC). A standardized data collection form was used to extract the following information: (i) study characteristics: study design, setting, countries and settings, funding; (ii) participant’s characteristics, sample size and type of trauma; (iii) intervention type and outcomes. The authors of the selected studies were contacted if the reported data were not reported in detail or were incomplete. We hand searched potential references from lists of included studies.
Internal validity
The internal validity of the included studies was assessed using the Cochrane Risk of Bias (RoB) tool for RCTs [16] and the Newcastle-Ottawa scales [17] for observational studies. The following domains of the Cochrane RoB tool were appraised: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective reporting) (58). Each domain was classified as “high”, “low” or “unclear” RoB if the study did not provide sufficient information to be classified.
In the Newcastle-Ottawa scales, the following domains were appraised: selection, comparability, outcome. Thresholds for converting the Newcastle-Ottawa scales to Agency for Healthcare Research and Quality standards (good, fair and poor) were adapted. Two reviewers (SG, GC) independently evaluated the methodological quality of the included studies; any disagreement was resolved by a consensus between reviewers.
Data synthesis
The treatment effects for dichotomized outcomes were evaluated using the odds ratio (OR), and when studies adopted strategies to minimize confounding factors (e.g. adjustment propensity score/multivariable analyses), the adjusted odds ratio (aOR) was adopted; for continuous outcomes, the pooled mean difference (MD), or standardized mean difference (SMD) for different outcome measurements, was used. The variance was expressed with 95% confidence intervals (95% CI). When applicable, the outcome measures from the individual trials were combined through meta-analysis using random-effects models described by DerSimonian and Laird [18] because a certain degree of heterogeneity of population and treatments would be expected among interventions. Both crude and adjusted pooled treatment effects were reported in tables as well as in forest plots. A subgroup analysis for every included comparison was finally planned in order to better answer different questions (vs RT, v. RT + REBOA, vs no-REBOA). All tests were considered statistically significant, for p values less than 0.05. The analyses were performed by using RevMan Version 5.4 (Nordic Cochrane Center) [19].
Quality of evidence
The quality of evidence of each outcome was judged through five dimensions (risk of bias, consistency of effect, imprecision, indirectness and publication bias) by the GRADE approach [20]. The evidence was downgraded from “high quality” by one level if serious, or by two levels if very serious limitations were found for each of the 5 dimensions. We presented a summary of findings describing the treatment effects, the quality of evidence and the reasons for limitations.
Results
Study selection
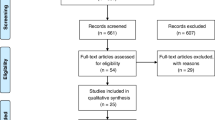
A total of 324 publications were selected for the analysis. No randomized controlled trials were found, one registered trial protocol not completed, five systematic reviews of observational studies and 10 observational studies met the eligibility criteria. A comparative evaluation between studies included in the systematic reviews and the primary studies resulting from the search strategy was performed: at the end of the search, 11 primary observational studies were included [21,22,23,24,25,26,27,28,29,30,31]. The flow diagram is reported in Supplement B.
General characteristics
Overall, five studies assessed the comparison REBOA vs RT [21,22,23,24, 29], one study reported the comparison REBOA vs RT + REBOA [28] and five studies investigated the comparison REBOA vs no-REBOA [25,26,27, 30, 31]. Two of the selected studies were prospective [23, 24] and nine were retrospective [21, 22, 25,26,27,28,29,30,31]. The median injury severity score (ISS) across studies ranged from a minimum of 25 (IQR: 16–25) [25] and a maximum of 44 (IQR: 38–59) [28]. Blunt trauma was the most representative feature of patients across studies, except for one study that included only patients with penetrating trauma [25]. General characteristics are reported in Table 1.
Overall mortality
All studies (n = 11) reported overall mortality data (Table 2). Most of these (n = 9) did not report the time from injury to death, similarly for the outcome at discharge without a specific time frame. In addition, five studies evaluated overall mortality in the emergency department, while four studies assessed the mortality at 24 h and three studies reported data at 1 month (Supplement C).
Crude summary estimates found statistically significant differences in favour of REBOA when compared to RT (OR 0.42; 95% CI 0.32–0.54, I2 = 0) or RT with REBOA (OR 0.13; 95% CI 0.04–0.47) and against REBOA when compared to no-REBOA (OR 0.68; 95% CI 1.03–2.72, I2 = 87). Adjusted summary estimates confirmed a statistically significant difference favouring REBOA vs. RT (aOR 0.38; 95% CI 0.20–0.74, I2 = 37), whereas no significant difference was present when REBOA was compared to no-REBOA (aOR 1.40; 95% CI 0.79–2.46, I2 = 90). Figure 1 a shows the crude pooled treatment effects, whereas Fig. 1 b shows the adjusted pooled treatment effects of overall mortality of the studies that provided data at discharge or at the last available follow-up adjusted by matching with the propensity score or by regression. Figure 2 shows mortality in the emergency department, while Fig. 3 presents 24-h mortality. Individual studies investigated mortality at 1 month for RT vs REBOA [28] (aOR not estimable) and REBOA vs. no-REBOA (aOR 0.77, 95% CI 0.36–1.61) [27, 30], without evidence of significant effects (Supplement C).
Volume of infused blood components
Six studies investigated the volume of blood components. Some studies demonstrated a statistically significant decrease in the number of plasma [24, 25] and platelets [27] infused to the patients and in the number of patients who needed transfusion [30]. However, heterogeneity in the reporting of outcome measurements prevents a quantitative analysis and data are reported in Table 3.
Health-related quality of life
The Discharge Glasgow Coma Scale (Discharge GCS) among survivors, as a proxy of health-related quality of life outcome, was reported in four studies [23, 24, 27, 31].
A study [24] found no significant differences between the 2 groups (median REBOA group, 15 points; median RT group, 15 points, p = 0.766) reported, and in another study [23], the Discharge GCS was in favour of the REBOA group, but only in the pre-hospital cohort (median REBOA group, 9 points; median RT group, 3 points, p = 0.026).
In the last two studies [27, 31] only a subgroup analysis in patients who received REBOA was performed. Surviving patients had higher Glasgow Coma Scale (GCS) than non-survivors in both Norii et al., 2015 (mean GCS, 11.6 survivors vs. 7.2 non-survivors, p = 0.0001) and Joseph et al. 2019 studies (median (IQR) GCS, 15 (13-15) survivors vs 3 (3-13) non-survivors, p = 0.04).
Adverse effects
Adverse events for both groups of treatment (REBOA vs. RT) were reported in three studies [23, 24, 27], whereas one study [25] reported adverse events only for the REBOA group. Overall, the most frequently reported complications from the studies were amputation, haematoma and pseudo-aneurysm, shown in Supplement C.
Other outcomes
Two studies reported the number of subjects in which the technique was successfully performed (> 91%) [23, 24]. Two studies reported the temporary time to control haemorrhage [27, 28]. One study [28], investigating REBOA vs REBOA +RT, reported the control time for bleeding from arrival at the scene. Patients with arterial access achieved within 21.5 min of arrival demonstrated immediate subsequent haemostasis. Heterogeneous measurements for the improvement in haemodynamics (blood pressure and heart rate) are reported in two studies [23, 24]. Supplement C descriptively reported data.
Internal validity and quality of evidence
Seven studies were judged to be of good quality and four of fair quality (Supplement D). Certainty of evidence ranged from very low to low with no serious risk of bias. We downgraded the evidence for serious indirectness and imprecision of the estimates (Supplement E).
Algorithm for decision-making
REBOA might be considered in haemodynamically unstable patients, unresponsive to initial resuscitation for a suspected torso haemorrhage, as indicated by E-FAST positive for free peritoneal fluid and/or pelvis x-ray indicating fracture of the ring. REBOA is inflated in zone 1 if positive E-FAST or impending cardiac arrest and zone 3 if pelvic fracture. REBOA is progressively deflated as soon as the bleeding site is controlled with temporary or definitive surgical techniques, while continuing volume replacement. REBOA is not indicated in the suspicion of injury of the thoracic aorta and if emergency room diagnostic tools fail to demonstrate a torso haemorrhage. Figure 4 describes an algorithm for REBOA indications.
Discussion
To our knowledge, this is the first comprehensive systematic review and meta-analysis assessing all comparisons between REBOA and RT and REBOA versus no-REBOA, considering many critical and important outcomes. With low quality of evidence, adjusted overall estimates found a difference in favour of REBOA when compared to RT (aOR 0.38; 95% CI 0.20–0.74). With very low quality of evidence, REBOA when compared to no-REBOA (aOR 1.40; 95% CI 0.79–2.46) did not show a significant difference in outcomes. Adverse events were poorly reported across studies: only four studies reported complications such as amputation, haematoma and pseudoaneurysm.
Our literature search found five systematic reviews about the use of REBOA. However, two investigated the REBOA in a variety of clinical settings [10, 32], and one focussed only on adverse events revising case series studies [33]. Our results are consistent with other two systematic reviews [34, 35] where the comparison of REBOA versus RT found similar quantitative findings (aOR 0.42; 95%CI 0.17–1.03; OR 0.25; 95%CI 0.11–0.56, respectively). Nevertheless, our review updated the evidence including the last three years of publication.
All the studies where REBOA was compared to RT [21,22,23,24, 29] demonstrated a clear survival benefit in very sick patients. This statistical significance can be biased since the most serious patients in cardiac arrest or imminent cardiac arrest undergo a RT, while more stable patients might be considered for REBOA. In very sick patients with very low critical tissue and organ perfusion or impending cardiac arrest, extreme resuscitative manoeuvres are required as a bridge to save time to definitive bleeding control or other potential reversible injury management. RT is a maximally invasive procedure in the trauma setting with a survival of less than 10% but associated with a negative perception because often used as the last resort in patients beyond saving [36, 37]. In this comparison, patient selection separates patients into the dead-or-nearly-dead (RT) and the alive-but-severe-shock (REBOA) with no valid conclusions regarding the superiority or preference of REBOA over RT. Therefore, in the absence of randomized trials, REBOA can only be considered an option for the clinician in very sick haemorrhagic patients following trauma. The more valuable contribution to the literature should come from the comparison of REBOA and no-REBOA. However, when REBOA has been compared with no-REBOA in our analyses [25,26,27, 30] a clinical benefit was not observed. The available published studies range from very low and low certainty of evidence, including patients with brain injuries or chest injuries on which REBOA has no benefit and sometimes can be harmful. Moreover, the evidence was limited to observational studies. In all studies, the clinical indication to REBOA was haemorrhage from a pelvic fracture or abdominal injury, conditions that recognize optimal clinical results with standard damage control approaches, such as abdominal or extraperitoneal packing [38]. Both manoeuvres improve haemodynamics and survival and are easily performed by surgical personnel with specific experience.
REBOA stops flow totally or partially below the occlusion level (zone I or III), inflation limits the bleeding but also does produce ischemia both regionally and systemically. Given the tendency towards reperfusion injury, REBOA has a limited time window of application before the complications overcome the benefit of intervention [39, 40]. REBOA is likely not clinically better than the standard and more consolidated damage control interventions for bleeding control in non-compressible torso haemorrhagic shock, but can be implemented as a life-saving haemostatic bridge, if damage control surgery is not immediately available after prompt evaluation and indications based on clinical characteristics of patients. Furthermore, many variables can affect survival in this category of patients: pre-hospital time, prompt recognition of bleeding site(s), availability of expert surgeon, time to operating room, appropriate transfusion protocol, physiology of the patient. Time to operating room and physiologic state of patients may predict outcome as importantly as does whether a REBOA is used [41]. Due to these considerations, further investigations with an adequate volume of cases considering all possible confounders can help to understand the efficacy of REBOA in torso haemorrhages.
Limitations
Our review is the most comprehensive effort in the management of haemorrhage in major trauma patients; however, several limitations must be addressed. Although our quantitative synthesis shows that REBOA is associated with lower mortality when compared to RT, these results could be flawed by the presence of patient selection for indication bias and survival bias within the individual observational studies [42]. Indication bias arises when patients are classified on the basis of the non-randomized intervention they received during the natural course of their medical treatment. Survival bias appears when comparing groups in which patients may die before treatment is initiated [43]. Clinical conditions (e.g. cardiac arrest) strongly influenced the treatment indication and so the assignment of patients in the RT or no-REBOA group. In the emergency department, RT is performed in patients who are experiencing post-traumatic cardiac arrest, while REBOA is indicated for trauma patients who are in an uncontrolled haemorrhagic shock for a pelvic fracture or abdominal fluid detected on an initial ultrasonography scan in the trauma bay [27]. For these reasons, some studies may have an inadequate control group (i.e. patients who did not undergo REBOA placement and/or RT). We have overcome this limitation by subgrouping the patients, who underwent thoracotomy in the ED, patients who underwent REBOA and those who did not undergo REBOA. Unfortunately, we did not find RCTs, the most reliable evidence on the effectiveness of interventions [44] which minimize the risk of bias and confounding factors influencing the results [45]. Even if performing RCTs can be unethical in life-threatening situations, challenging to design and deliver it is not impossible: a recent mapping review has highlighted that evidence from trials in prehospital trauma is sparse and can be prioritized [46]. We call for the need for further randomized trials of REBOA vs RT and REBOA vs no-REBOA in order to assure well-matched patients.
The use of REBOA should take into account skills, high expertise on their applicability [47, 48], acceptability of clinicians and cost [49, 50]. For optimal success, REBOA requires careful system-wide multidisciplinary implementation [51]. Institutions are responsible for analysing qualifications for providers to perform REBOA [42] as well as evaluating system capabilities [52]. A very small number of trauma centres have an extensive experience with REBOA; thus, these results may not be generalizable to all trauma centres [42]. Finally, we included studies with a heterogeneous use of REBOA which should be taken into account (catheter size, occlusion zone, protocols, physiologic indications for REBOA insertion).
Conclusion
Among non-compressible torso injuries, we found a positive effect on overall mortality of REBOA when compared to RT but no valid conclusions can be made due to selection bias, while not statistically significant the comparison of REBOA versus no-REBOA from which the most valuable contribution for clinical practice is drawn. REBOA should be promoted in specific training programs in an experimental setting in order to test its effectiveness as temporary management to haemorrhage control and resuscitation. Prospectively assessed data, with specific inclusion and exclusion criteria, ideally in a randomized controlled trial, should be planned in order to limit the bias coming from observational studies. Future studies must address specific indications for REBOA to know which population could benefit the most from its use.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its additional files]. Row data are stored in an open platform at the following link: https://osf.io/ntxvj/
Abbreviations
- aOR:
-
Adjusted odds ratio
- GCS:
-
Glasgow Coma Scale
- IQR:
-
Interquartile range
- ISS:
-
Injury severity score
- MD:
-
Mean difference
- MOOSE:
-
Meta-Analysis of Observational Studies in Epidemiology
- OR:
-
Odds ratio
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT:
-
Randomized controlled trials
- REBOA:
-
Resuscitative Endovascular Balloon Occlusion of the Aorta
- RoB:
-
Cochrane Risk of Bias
- RT:
-
Resuscitative thoracotomy
- SMD:
-
Standardized mean difference
- SNLG:
-
Sistema Nazionale Linee Guida
References
Andres J, Scott J, Giannoudis PV. Resuscitative endovascular balloon occlusion of the aorta (REBOA): what have we learned? Injury. 2016;47(12):2603–5.
Rotondo MF, Schwab CW, McGonigal MD, Phillips GR 3rd, Fruchterman TM, Kauder DR, et al. 'Damage control': an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35(3):375–82 discussion 82-3.
Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surgery. 2015;78(5):1054–8.
Cannon J, Morrison J, Lauer C, Grabo D, Polk T, Blackbourne L, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) for hemorrhagic shock. Mil Med. 2018;183(suppl_2):55–9.
McGreevy DT, Sadeghi M, Pirouzram A, Toivola A, Dogan EM, Larzon T, et al. Feasibility and clinical outcome of Reboa in patients with impending traumatic cardiac arrest. Shock. 2019;16:1540–0514.
Saito N, Matsumoto H, Yagi T, Hara Y, Hayashida K, Motomura T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surgery. 2015;78(5):897–903 discussion 4.
Fitzgerald M, Mathew J, Yeung M, Niggemeyer L, Hendel S. https://orcid.org IO, et al. feasibility study for implementation of resuscitative balloon occlusion of the aorta in peri-arrest, exsanguinating trauma at an adult level 1 Australian trauma Centre. Emerg Med Aust. 2020;32(1):127–34.
Lendrum R, Perkins Z, Davies G, Chana M, Marsden M, Davenport R, et al. Pre-hospital resuscitative endovascular balloon occlusion of the aorta ( REBOA ) for exsanguinating pelvic haemorrhage. Resuscitation. 135:6–13.
Ordonez Carlos A, Rodriguez F, Serna Jose J, Salcedo A, Del Valle AM, Garcia A, et al. Resuscitative endovascular balloon of the aorta is feasible in penetrating chest trauma with major hemorrhage : proposal of a new institutional deployment algorithm. J Trauma Acute Care Surg. 2020;27(27):2163–0763.
Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg. 2016;80(2):324–34.
SNLG, Sistema Nazionale Linee Guida, Linea Guida sulla Gestione Integrata del Trauma Maggiore dalla scena dell’evento alla cura definitiva https://snlg.iss.it/?p=2533, accessed 20 Jan 2021.
Schünemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, et al. GRADE evidence to decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101–10.
Iannone P, Coclite D, Napoletano A, Fauci A. Manuale metodologico per la produzione di linee guida di pratica clinica: Centro Nazionale per l’Eccellenza Clinica, la Qualità e la Sicurezza delle Cure dell’Istituto Superiore di Sanità. 2018 Available from https://snlg.iss.it/wp-content/uploads/2019/04/MM_v1.3.2_apr_2019.pdf.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Medicine. 2009;3(3):e123–30.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Wells GA SB, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp accessed 30 October 2020. 2010.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Abe T, Uchida M, Nagata I, Saitoh D, Tamiya N. Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: a nationwide cohort study in Japan. Crit Care. 2016;20(1):400.
Aso S, Matsui H, Fushimi K, Yasunaga H. Resuscitative endovascular balloon occlusion of the aorta or resuscitative thoracotomy with aortic clamping for noncompressible torso hemorrhage: a retrospective nationwide study. J Trauma Acute Care Surg. 2017;82(5):910–4.
Brenner M, Inaba K, Aiolfi A, DuBose J, Fabian T, Bee T, et al. Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the American Association for the Surgery of Traumaʼs aortic occlusion in resuscitation for trauma and acute care surgery registry. J Am Coll Surg. 2018;226(5):730–40.
DuBose JJ, Scalea TM, Brenner M, Skiada D, Inaba K, Cannon J, et al. The AAST prospective aortic occlusion for resuscitation in trauma and acute care surgery (AORTA) registry: data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surgery. 2016;81(3):409–19.
Garcia Alberto F, Manzano-Nunez R, Sanchez Alvaro I, Ordonez Carlos A, Garcia Alberto F, Melendez J, et al. Association of resuscitative endovascular balloon occlusion of the aorta ( REBOA ) and mortality in penetrating trauma patients. Eur J Trauma Emerg Surg. 2020;16(16):1863–9941.
Inoue J, Shiraishi A, Yoshiyuki A, Haruta K, Matsui H, Otomo Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: a propensity score analysis. J Trauma Acute Care Surgery. 2016;80(4):559–66 discussion 66-7.
Joseph B, Zeeshan M, Sakran JV, Hamidi M, Kulvatunyou N, Khan M, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg. 2019;154(6):500–8.
Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Funabiki T. Investigators D-I. partial occlusion, conversion from thoracotomy, undelayed but shorter occlusion: resuscitative endovascular balloon occlusion of the aorta strategy in Japan. Eur J Emerg Med. 2018;25(5):348–54.
Moore LJ, Brenner M, Kozar RA, Pasley J, Wade CE, Baraniuk MS, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surgery. 2015;79(4):523–30 discussion 30-2.
Yamamoto R, Cestero RF, Suzuki M, Funabiki T, Sasaki J. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: a propensity score matching analysis. Am J Surg. 2019;218(6):1162–8.
Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surgery. 2015;78(4):721–8.
Gamberini E, Coccolini F, Tamagnini B, Martino C, Albarello V, Benni M, et al. Resuscitative endovascular balloon occlusion of the aorta in trauma: a systematic review of the literature. World J Emerg Surg. 2017;12:42.
Manzano-Nunez R, Orlas CP, Herrera-Escobar JP, Galvagno S, DuBose J, Melendez JJ, et al. A meta-analysis of the incidence of complications associated with groin access after the use of resuscitative endovascular balloon occlusion of the aorta in trauma patients. J Trauma Acute Care Surgery. 2018;85(3):626–34.
Manzano Nunez R, Naranjo MP, Foianini E, Ferrada P, Rincon E, Garcia-Perdomo HA, et al. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J Emerg Surg. 2017;12:30.
Borger van der Burg BLS, van Dongen T, Morrison JJ, Hedeman Joosten PPA, DuBose JJ, Horer TM, et al. A systematic review and meta-analysis of the use of resuscitative endovascular balloon occlusion of the aorta in the management of major exsanguination. Eur J Trauma Emerg Surg. 2018;44(4):535–50.
Rabinovici R, Bugaev N. Resuscitative thoracotomy: an update. Scand J Surg. 2014;103(2):112–9.
Suzuki K, Inoue S, Morita S, Watanabe N, Shintani A, Inokuchi S, et al. Comparative effectiveness of emergency resuscitative thoracotomy versus closed chest compressions among patients with critical blunt trauma: a nationwide cohort study in Japan. PLoS One. 2016;11(1):e0145963.
Frassini S, Gupta S, Granieri S, Cimbanassi S, Sammartano F, Scalea TM, et al. Extraperitoneal packing in unstable blunt pelvic trauma: a single-center study. J Trauma Acute Care Surgery. 2020;88(5):597–606.
Qasim ZA, Sikorski RA. Physiologic considerations in trauma patients undergoing resuscitative endovascular balloon occlusion of the aorta. Anesth Analg. 2017;125(3):891–4.
Hoareau GL, Tibbits EM, Beyer CA, Simon MA, DeSoucy ES, Faulconer ER, et al. Resuscitative endovascular balloon occlusion of the aorta: review of the literature and applications to veterinary emergency and critical care. Front Vet Sci. 2019;6:197.
Vella MA, Dumas RP, DuBose J, Morrison J, Scalea T, Moore L, et al. Intraoperative REBOA: an analysis of the American Association for the Surgery of Trauma AORTA registry. Trauma Surg Acute Care Open. 2019;4(1):e000340.
Bulger EM, Perina DG, Qasim Z, Beldowicz B, Brenner M, Guyette F, et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: a joint statement from the American College of Surgeons Committee on trauma, the American College of Emergency Physicians, the National Association of emergency medical services physicians and the National Association of emergency medical technicians. Trauma Surg Acute Care Open. 2019;4(1):e000376.
del Junco DJ, Fox EE, Camp EA, Rahbar MH, Holcomb JB, Group PS. Seven deadly sins in trauma outcomes research: an epidemiologic post mortem for major causes of bias. The journal of trauma and acute care surgery. 2013;75(1 Suppl 1):S97–103.
Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. Bjog. 2018;125(13):1716.
Akobeng AK. Understanding randomised controlled trials. Arch Dis Child. 2005;90(8):840–4.
Björklund MK, Cruickshank M, Lendrum RA, Gillies K. Randomised controlled trials in pre-hospital trauma: a systematic mapping review. Scand J Trauma Resusc Emerg Med. 2021;29(1):65.
Engberg M, Taudorf M, Rasmussen NK, Russell L, Lönn L, Konge L. Training and assessment of competence in resuscitative endovascular balloon occlusion of the aorta (REBOA) - a systematic review. Injury. 2020;51(2):147–56.
Theodorou CM, Salcedo ES, DuBose JJ, Galante JM. Hate to burst your balloon: successful REBOA use takes more than a course. J Endovasc Resusc Trauma Manag. 2020;4(1):21–9.
Renna MS, van Zeller C, Abu-Hijleh F, Tong C, Gambini J, Ma M. A one-year cost-utility analysis of REBOA versus RTACC for non-compressible torso haemorrhage. Trauma. 2019;21(1):45–54.
Nowadly CD, Johnson MA, Hoareau GL, Manning JE, Daley JI. The use of resuscitative endovascular balloon occlusion of the aorta (REBOA) for non-traumatic cardiac arrest: a review. J Am Coll Emerg Physicians Open. 2020;1(5):737–43.
Zakaluzny SA, Beldowicz BC, Salcedo ES, DuBose JJ, Moore LJ, Brenner M. Guidelines for a system-wide multidisciplinary approach to institutional resuscitative endovascular balloon occlusion of the aorta implementation. J Trauma Acute Care Surgery. 2019;86(2):337–43.
Napolitano LM. Resuscitative endovascular balloon occlusion of the aorta: indications, outcomes, and training. Crit Care Clin. 2017;33(1):55–70.
Acknowledgments
Italian National Institute of Health guideline working group on Major Trauma: Nino Stocchetti, Elvio De Blasio, Gaddo Flego, Massimo Geraci, Giulio Maccauro, Antonio Rampoldi, Federico Santolini, Claudio Tacconi, Gregorio Tugnoli. We would like to thank Maurella Della Seta, Scilla Pizzarelli, Rosaria Rosanna Cammarano, the Istituto Superiore di Sanità documentalists for performing the search strategy, and Alessia Medici and Alessandro Mazzola for the administrative and organizational support.
Funding
The work was supported by the Istituto Superiore Sanità with no financial funding, that played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
Greta Castellini: study design, data selection, data extraction, data analysis and drafting of the work and final approval of the version to be published with agreement in all parts; Silvia Gianola: study design, data selection, data extraction, data analysis and drafting of the work and final approval of the version to be published with agreement in all parts; Andrea Fabbri: interpretation of data for the work, gave inputs for intellectual clinical concepts and final approval of the version to be published with agreement in all parts; Annalisa Biffi: study design, data analysis and drafting of the work and final approval of the version to be published with agreement in all parts; Carlo Coniglio: interpretation of data for the work and revision for intellectual clinical concepts and final approval of the version to be published with agreement in all parts; Daniela Coclite: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Daniela D’Angelo: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Alice Josephine Fauci: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Shailvi Gupta: interpretation of data for the work and revision for intellectual clinical concepts and final approval of the version to be published with agreement in all parts; Laura Iacorossi: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Roberto Latina: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Antonello Napoletano: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Gloria Porcu: study design, data analysis and drafting of the work and final approval of the version to be published with agreement in all parts; Maria Pia Ruggeri: interpretation of data for the work and inputs for intellectual clinical concepts and final approval of the version to be published with agreement in all parts; Katia Salomone: critical review and editing of the work, interpretation of data and final approval of the version to be published with agreement in all parts; Primiano Iannone: conceived idea, interpretation of data for the work and revision of critically for important intellectual content and final approval of the version to be published with agreement in all parts; Osvaldo Chiara: conceived idea, interpretation of data for the work, and revision of critically for important intellectual content and final approval of the version to be published with agreement in all parts.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Castellini, G., Gianola, S., Biffi, A. et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in patients with major trauma and uncontrolled haemorrhagic shock: a systematic review with meta-analysis. World J Emerg Surg 16, 41 (2021). https://doi.org/10.1186/s13017-021-00386-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13017-021-00386-9