Abstract
Background
Maternal rectovaginal colonization with Streptococcus agalactiae (Group B Streptococcus or GBS) is the most common route for the GBS disease in the perinatal period. The knowledge of maternal colonization, antibiotic resistance and serotype profiles is substantially needed to formulate the broad vaccine. However, it has not been estimated in Africa. This meta-analysis was aimed to determine the pooled prevalence of colonization, antibiotic resistance and serotype profiles of GBS reported in Africa.
Methods
Potentially relevant studies from 1989 to 31th January, 2019 were retrieved from the Medline/PubMed, EMBASE, HINARI online databases, periodicals and by requesting authors. Unpublished studies retrieved from grey literature through Google and Google Scholar. Pooled estimates were calculated using the random effect model. Subgroup analysis was done to investigate the burden of colonization across sub-regions, sampling site and countries. Summary estimates were presented using words, Forest plots and Tables. Heterogeneity was assessed using the I2 statistic.
Results
Eighty-three articles were assessed, of which 57 studies conducted in five sub-regions with 21 countries (22,206 pregnant women) met pre-specified inclusion criteria. The overall estimate of recto-vaginal colonization was 19.3% (95% CI 16.9, 21.7). The highest estimate was observed in Southern Africa, 23.8% (95% CI 18.7, 28.9), followed by Northern Africa, 22.7% (95% CI 18.2, 27.2) while the lowest was driven from the Eastern Africa, 15.4% (95% CI 12.1, 18.7). Considerable heterogeneity across and within regions, sampling site, screening methods and countries (I2 > 75%); and the publication bias were observed (p = 0.031). GBS showed the highest resistance to tetracycline. Resistance to penicillin, amoxicillin, chloramphenicol, ampicillin, ceftriaxone, ciprofloxacin, erythromycin, vancomycin and clindamycin also observed. The V, III, Ia, Ib, and II serotypes altogether were accounted 91.8% in the African studies.
Conclusions
The pooled estimate of the maternal colonization with GBS was 19.3% which is equivalent with other many primary and review reports worldwide. The most antibiotic resistance estimate was recorded in the tetracycline followed by penicillin. Five serotypes were the most prevalent in Africa and more data on the antibiotic résistance and serotype distribution patterns are needed from developing countries to devise the effective preventive measures. In addition, the antibiotic susceptibility test methods used in the Africa shall be assessed for its quality.
Trial registration Prospero Registration Number CRD42018094525
Similar content being viewed by others
Background
Streptococcus agalactiae (or S. agalactiae or Group B Streptococcus; GBS) is one of the many serologically distinct species within the genus Streptococcus [1, 2]. It is an encapsulated diplococcus exhibiting ß-haemolysis on blood agar, facultative anaerobe, nutritionally fastidious, catalase, and mannitol salt negative. It also hydrolyzes sodium hippurate, bacitracin resistant, CAMP test positive and chain forming group. It is found as a commensal organism in the gut and genital tract of both female and male healthy adults. It causes severe illnesses in people of all ages, ranging from bloodstream infections (sepsis) and pneumonia to meningitis and skin infections [1, 3]. It also causes a significant agricultural and veterinary problem, since it can infect the ruminants` mammary glands [4], and fishes [5].
In the 1970s, GBS was the dominant pathogen in the early neonatal period [6]. It also became the most common cause of neonatal sepsis and meningitis in many developed countries in the early 1980s [7]. Newborns from GBS colonized mothers could be exposed in utero, or during delivery as they swallow or aspirate the bacterium while passing through the birth canal. GBS infection in infants causes sepsis and meningitis which could result in acute illness, long-term disabilities and death [8]. Isolates from human express capsular polysaccharide (CPS), a major virulence factor that helps the bacterium to evade the host defense mechanisms [9].
Primary studies conducted in the East African countries showed the colonization rates ranged from 3.0% to 28.8% [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]; Central Africa, 20.0% [27, 28]; Western Africa, 2.5% to 34.2% [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]; Southern Africa, 1.77% to 48.23% [49,50,51,52,53,54,55,56,57,58,59,60,61]; and Northern Africa, 17.00% to 26.5% [62,63,64]. GBS isolated from pregnant women in different primary studies conducted in Africa showed resistance to penicillin, ampicillin, erythromycin, clindamycin, vancomycin, ciprofloxacin, chloramphenicol, and tetracycline [10, 11, 15, 31, 36, 65]. Unlike to Western countries, few data are available about GBS serotypes in different parts of the Africa since the 1989 in Ethiopia to 2018 in Morocco [22, 28, 30, 31, 33, 39, 49, 54, 66]. A review from the USA on GBS serotypes showed lower proportions of women with serotypes Ia, Ib, or III with the mean prevalence estimate of 55.0%, and in Europe, 58.3% [67].
A systematic review done 10 years ago on 21 studies included 24,093 women from the 13 European countries indicated that GBS colonization varied from 6.5% in Turkey to 36% in Denmark [68]. Another recent review, based on the studies using the recommended methods, estimated the maternal GBS prevalence as 17.9% worldwide, ranging from 11.1% in Southeast Asia to 22.4% in Africa [67]. Such a review included 78 primary studies from the 37 countries with main limitations in Africa and Asia. Another meta-analysis study included 390 articles from 85 countries with a total of 299, 924 pregnant women showed 18% overall global estimates of maternal GBS colonization, with regional variation from 11.1 to 34.7%, and lower prevalence in Southern Asia, 12.5% and Eastern Asia, 11% [69].
Reviews of the prevalence estimate of pregnant women colonized with GBS, antibiotic resistance profile and serotype distribution are useful to generate evidence and to devise the preventive measures. Thus, this review was aimed to estimate the pooled prevalence of maternal colonization with GBS, antibiotic resistance and serotype patterns reported from various studies conducted in African countries.
Methods
Identification and selection of studies
Published and unpublished research reports describing GBS maternal colonization, antibiotic resistance profile and serotype distribution in Africa since 1989 to 31th January, 2019 were reviewed. Potentially relevant studies were identified through a literature search of PubMed/Medline, HINARI, and EMBASE online databases; from periodicals to requesting articles from publishers/authors. Unpublished studies were retrieved from the grey literature through Google and Google Scholar. All searches were limited to English language and conducted from February 2018 to January 2019. The phrase ‘Streptococcus agalactiae’ was searched following a combination of free text and thesaurus terms in different variations: Group B Streptococcus, GBS, Streptococci, maternal, pregnancy, parturient, third trimester, colonization, carriage, vaginal, rectal, vaginorectal, rectovaginal, prevalence, proportion, antibiotic/drug/antimicrobial, resistance/susceptibility patterns/profiles, serotype, serotype distribution, and Africa. The following keywords were used to retrieve studies from PubMed database; (Streptococcus agalactiae) AND (maternal AND colonization OR (parturient AND prevalence AND proportion)) AND (antibiotic/antimicrobial susceptibility/resistance AND serotype OR (drug AND resistance)) AND (Africa). The search was carried out by three authors (MG, MT, & FM), the most relevant studies were selected using predefined inclusion and exclusion criteria. The last author (BT) has checked the overall consistency of the searching process, study choice and inclusion/exclusion criteria.
Abstracts were reviewed from a first search using predefined inclusion and exclusion criteria. Original studies from the African settings were included in this systematic review and meta analysis study, whereas comments, editorials, and reviews were excluded. The articles were included if they estimated the proportion/prevalence/carriage and/or antibiotic resistance patterns and/or the serotype profiles among the pregnant women colonized with GBS; excluding those colonized mothers for whom proportion of colonization were not reported. The review was carried out by using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline (Fig. 1) [70] records after duplicates were removed.
Data extraction
Two authors (MG, FM) performed data abstraction using excel spreadsheet form. These authors independently examined titles, abstracts, full-text articles, and abstracted data using the same data abstraction forms and selection criteria from studies conducted on maternal GBS colonization in Africa since 1989 to 31th January, 2019. Disagreements were resolved by consensus among these investigators. The third and fourth authors, MT and BT arbitrated any discrepancies between the two authors who primarily abstracted the data. From each study, the following parameters were extracted: numbers of pregnant women involved in the study, culture methods used, specimen collection site, colonization (GBS positive), antibiotic resistance and serotype profiles of the isolates. Moreover, the authors retrieved data on study country, sub-region/continent, study year, and study design. Ethical approval for this review was not applicable.
Validity assessment
Studies were assessed for quality, with moderate to high quality studies included in the analysis. The quality of included studies was assessed by using the Newcastle–Ottawa quality assessment scale [71]. Two authors (MG, MT) independently assessed the methodological quality, quality of reported data (extractable data to calculate colonization proportion, antibiotic resistance profile and serotype distribution and cleared data research design of the included studies. After assessing the quality of each study included on the basis of these criteria, a composite quality score was assigned, ranging from 0 to 7. Studies scoring 5 and above were judged to be of moderate to high quality.
Data analysis
The data extracted were entered into the Microsoft excel spreadsheet and were exported to the STATA version 14 (Stata Corp LLC, Texas, USA) for analysis. The magnitude of heterogeneity between the included studies was quantitatively measured by an index of heterogeneity (I2 statistics) [72]. The low, medium and high heterogeneity were represented as the I2 values of 25%, 50% and 75%, respectively. The statistical significance of heterogeneity was determined by a p-value of I2 statistics. A p-value ≤ 0.05 statistically showed heterogeneity. If I2 value was greater than 50%, we used Dersimonian and Liard random effect model to determine the pooled estimates of GBS colonization proportion, antibiotics resistance and serotype profiles of the isolate [73]. The subgroup analysis was conducted by considering sub-regions, countries, specimen collection sites and method of GBS screening used as a grouping variable (Table 1). Small-study effects and publication bias were evaluated first visually by using the funnel plot (Fig. 4), and then by Egger’s statistics in the random effect model (Table 2). The p-value ≤ 0.05 was considered indicative of the presence of statistically significant publication bias [74,75,76,77] quantified. The trim and fill method was used to correct the publication bias as indicated in Fig. 5. The results were presented in text, tables, funnel and Forest plots.
Outcome of interest
The major outcome of interest of this review was the pooled proportion of GBS colonization of pregnant women, antibiotic resistance profiles and serotype patterns of the isolates reported from different studies in Africa. Sub-group analysis was done by sub-regions (Northern Africa, Western Africa, Central Africa, Eastern Africa and Southern Africa), and the 21 countries as detailed in Table 1. The proportion of resistance GBS to the 10 different antibiotics was calculated by dividing the numbers of resistance isolates by the total number of GBS isolated from pregnant women. The proportion of 10 capsular type patterns of GBS was also carried out by using the methods which we applied for the estimate analysis of antibiotic resistance proportion.
Results
This meta-analysis study pooled the colonization, antibiotic resistance profiles and the serotype distributions of GBS isolates which have investigated in small and fragmented ways. As shown in Fig. 1, 57 studies were identified from the five sub-regions of the African continent. These studies included 22,206 pregnant women for the estimation of maternal GBS colonization proportion, 1974 GBS isolates were tested for antibiotic susceptibility profiles, and 2223 GBS isolates were analyzed for serotype distribution. The pooled estimate of the maternal GBS colonization proportion in this study was 19.3% (95% CI (16.9, 21.7) (Table 1, and Fig. 2).
Among the 22,206 pregnant women included in 57 studies across the 21 countries, 4564 (3393 rectovaginal and 1171 vaginal) pregnant women were colonized with GBS (Tables 1, 2).
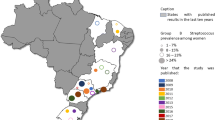
Considerable heterogeneity was observed in this meta-analysis (I-squared, 95.6%). To find the possible source (s) of variability between the included studies in this review, sub-group analysis was done by using five sub-regions, the 14 studies from the five Southern African countries had the highest number of pregnant women (n = 8849) participated in the study while the two studies conducted in the Central African countries had (n = 1058) the lowest number of the study participants. The overall mean proportion estimates of 19.3% (95% CI 16.9, 21.7) were slightly similar to the estimate derived from the Central African studies 19.9% (95% CI 17.5, 22.3) (Table 1 and Fig. 3). In addition, the highest colonization proportion was estimated from studies compiled in the Southern African countries, 23.8% (95% CI 18.7, 28.9), followed by studies conducted in Northern African courtiers, 22.7% (95% CI 18.2, 27.2). While the least estimate of maternal GBS colonization proportion was observed from the East African studies, 15.4% (95% CI 12.1, 18.7) (Table 1 and Fig. 3). Among the GBS screening techniques used in studies conducted in the African countries, the rapid test method accounted the highest estimate (23.2%, 95% CI 10.7, 35.6) though the estimate was derived from two studies while the direct plating techniques had the lowest estimation (14.7%, 95% CI 11.4, 17.9) (Table 1).
As detailed in Table 2, the percentage of laboratories employed by the primary author (s) were detailed by the five sub-regions, samples used and 21 countries where the 57 studies (primary articles) compiled to assess whether the differences or heterogeneity of colonization prevalence observed are attributable to geographical, methodological or sample types used differences. South Africa among the 14 studies, 11 (78.6%) used the broth enrichment techniques prior to inoculating on to solid media, followed by West Africa, 14 (70.0%). Studies from Central Africa used direct plating method. In all sub-regions, the primary authors used more recto-vaginal samples for GBS screening, and the highest estimate was recorded among the Sothern African countries, 12 (85.7%), followed by the East Africans, 13 (76.5%).. Six countries which contributed ≥ 3 articles had 36 (63.2%) article coverage for this study. Of these countries, South Africa and Ghana used 100% enrichment broth followed by Nigeria (90.0%). Twelve studies from 10 countries failed to use the prior enrichment techniques for GBS screening, and four studies collected from four countries also did not use recto-vaginal samples. Table 2 also showed us that the sub-regions which used the prior enrichment broth (70.0%) and recto-vaginal samples (74.3%) had better detection rates of GBS. It was also reflected in the countries at which more GBS was recovered by using prior enrichment broth (72.5%), and recto-vaginal sample (74.3%).
Further more, small study effect (or publication bias) was observed in this review as it is shown in the funnel plot (Fig. 4) and Egger’s statistical test (p-value = 0.031) (Table 3).
Thus, trim and fill method was used to correct publication bias observed in our meta-analysis and the corrected symmetric graph is indicated in Fig. 5.
Antibiotic resistance profiles of Group B Streptococcus
As detailed in Fig. 1 and Table 4, of the 57 studies collected from 21 African countries, the 35 studies reported the antibiotic resistance patterns of GBS among the 1974 isolates obtained from pregnant women. The highest pooled proportion of antibiotic resistance was observed in Tetracycline, 82.6% (95% CI 75.9, 89.4), followed by penicillin, 33.6% (95% CI 17.0, 50.1).
Serotype distribution
Of the 57 articles reviewed, 15 studies had serotype analysis of GBS in Africa from the dataset (Fig. 1). At least one of the ten serotypes was identified among these 15 studies included in this review, including the non-type-able (NT) isolates in nine studies. The pooled proportion of serotypes of maternal GBS analyzed from the five or more studies indicated that serotype V, III, Ia, Ib and II, accounted 91.8% coverage in the African reports (Table 5) that reported in ten or more studies. These serotypes were predominant in the African setting each with: 29.2% (95% CI 19.8, 38.6), 19.7% (95% CI 10.9, 28.5), 17.6% (95% CI 11.9, 23.4), 15.6% (95% CI 10.7, 20.5), and 12.4% (95% CI 8.4, 16.4) respectively.
Discussion
So far the prevalence of maternal colonization with GBS, antibiotic resistance profiles and serotype distributions of the isolates in the African setting is investigated in small and fragmented ways. Therefore, this is the first meta-analysis of its kind to summarize the pooled proportion of maternal recto-vaginal GBS colonization reported in 57 studies among 21 countries. Hence, in the present analysis, the pooled estimate of the colonization proportion was 19.3% (95% CI (16.9, 21.7) with the sub-regional variation of 14.0% (95% CI 10.41, 17.60) in Eastern Africa to 23.8% (95% CI 18.7, 28.9) in the Southern Africa (Table 1). Finding of the current meta-analysis is almost comparable with the meta-analysis study conducted worldwide in the 2016 which had 17.9% (95% CI 16.2, 19.7) overall estimates of maternal rectovaginal GBS colonization proportion from 78 studies with 73,791 pregnant women [67]. However, in the sub-group analysis of such a global estimate of the maternal rectovaginal colonization, the Africa represented in only four studies involving 2735 participating women with 619 GBS positive took the highest estimate of colonization proportion, 22.4% compared to the other sub-groups in such a review. This pooled result is slightly higher than the overall estimate of the colonization proportion of the current meta-analysis. This discrepancy might be explained by the variability in the number of the studies involved in the meta-analysis, the number of pregnant women participated in between the two reviews, variations in the detection techniques (laboratory facilities) employed and biological factors among the study participants across the world.
Another meta-analysis study analyzed the dataset about maternal colonization included 390 articles, 85 countries, and a total of 299, 924 pregnant women found the worldwide adjusted estimate for maternal GBS colonization was 18% (95% CI 17–19), with the regional variations from 11.1% (95% CI 9.9–12.4) in the Eastern Asia to 34.7% (95% CI 29.5–39.9) in the Caribbean. In the same meta-analysis study, Africa represented in the 19 studies included 36,130 pregnant women with the reported prevalence rate of 18.2% (95% CI 16.1–20.4) and overall pooled estimate of the adjusted colonization proportion was 21.3% (95% CI 18.5–24.2) [69] which is in consistent with our estimate. In the Russell et al., review, the lowest estimate was recorded from the six studies analyzed from the Western Africa, 17.5% (95% CI 10.8–24.1) while the highest estimate was from the Southern Africa, 28.9% (95% CI 26.6–31.2). The current systematic review and meta-analysis which we analyzed also reaffirmed that the highest pooled proportion of maternal colonization was derived from the 14 studies compiled in the Southern African countries (23.8%) while the lowest estimate was from the 17 studies recorded from the Eastern African countries (15.4%) where higher estimate (19.4%) was made in the previous study [69]. The mean proportion of maternal colonization with GBS estimated in our study from West Africa (18.7%) was the same as the estimate of the previous review in the same (17.5%) sub-region analysis [69]. Furthermore, findings of the current review in Africa is lower than a sub-analysis of the systematic review done 10 years back on 21 studies presented data on 24,093 women from 13 European countries which indicated that GBS colonization was varied from 6.5% in Turkey, to 36% in Denmark [68]. In such a report, colonization estimate from Denmark is higher than the estimate of our meta-analysis, whereas the estimate from Turkey is lower than the estimate of the current study.
The possible reason for the inconsistency of the estimates between sub-regions might be associated with differences in the numbers of the studies (articles) analyzed (Table 2), variations in the number of the study participants (pregnant women) included in the studies, differences in GBS detection techniques and sample types (Table 2) used across the sub-regions where certain laboratories use different alternative tests available like prior enrichment broth or rapid tests while others use routine or traditional (direct plating of the swabbed specimen onto solid media) laboratory techniques, disparities in site of specimen collection, and biological factors of the study participants. This is supported by the findings of this study to which to find the possible sources of heterogeneity, sub-group analysis was conducted by considering sub-regions, site of specimen collections (type of samples), the laboratory methods used for and the countries where studies collected as a grouping variable and the result showed that there was considerable heterogeneity (Table 1) in most cases (I2 > 75%; p-value ≤ 0.05). The rapid GBS screening method gave the highest pooled estimate prevalence of pregnant women colonization with GBS (23.2% (95% CI 10.7, 35.6), but the result was estimated from the two reports (one was screened by PCR and the other one was by rapid test kit). Based on Table 2 details, we realized that use of prior enrichment broth and recto-vaginal samples contributed for the variability of the colonization prevalence estimates. In addition, the number of articles compiled may have an effect on this heterogeneity. Since the possible source of variations might be numerous, further studies are required to identify specific aspects of season, co-morbidities, ethnicity, genetics/biological factors, lifestyle, behavior or cultural practices that may be factors for increase in the prevalence of GBS in different geographical locations.
The widespread IAP uses to prevent early onset GBS disease has raised a concern about the emergence of antibiotic resistance among GBS isolates. GBS continues to be susceptible to penicillin, ampicillin, and first-generation cephalosporins [78,79,80,81]. However, the isolates with increasing minimum inhibitory concentrations (MICs) to penicillin or ampicillin have been reported in both the noninvasive [82] and invasive isolates [83]. A report revealed that a penicillin-binding protein (PBP2X) alterations were found in noninvasive GBS isolates [82]. Correspondingly, the pooled finding of the current review recorded the presence of a concern about the antibiotic resistance of GBS isolated from the pregnant women. The isolates exhibited resistance to penicillin, ampicillin, vancomycin, clindamycin and erythromycin, which are usually recommended for the IAP to pregnant women at near (4 h before) delivery [3]. Similarly, in a 2017 study conducted in USA, a minimum inhibitory concentration technique was used for susceptibility profile by micro-dilution test, showed the presence of 28 GBS isolates resistant to the six beta-Lactam antibiotics [84] though the prevalence varies.. In another study conducted in 2014, two isolates sent to the CDC Streptococcus Laboratory were confirmed as vancomycin resistant and both were grouped as capsular serotype II, multilocus sequence type 22 GBS [85]. In our review, the capsular serotype II is one among the dominant serotypes in the African setting.
Occurrence of resistant GBS isolates to the bet-lactam antibiotics and vancomycin in the low income countries including Africa is possibly because of the widespread use of the antibiotics empirically for the treatment of different infectious diseases and the availability of these drugs non-restrictively in different areas with lower price enable self prescription. This expanded use of the beta-lactam antibiotics in the treatment of several infective clinical syndromes and the easy of purchase over the counter might be the contributing causes for the selective pressure for the emergence of GBS resistance strains in the area. The resistant rates to vancomycin reported in different African countries also might have an epidemiologic link with serotypes II and sequence type 22 which needs further studies by using the high tech laboratory facilities. In addition, there is a need to devise a system that could help, at least, to decrease the irrational use of these antibiotics in Africa. From this review, we realized that the presence of resistance GBS to penicillin, ampicillin and vancomycin in the African studies is not uncommon in contrary to the reports from the western countries. Thus, evaluating the quality of GBS antimicrobial testing methods and the quality of the antimicrobial disks used including shipment and storage in the developing continents shall be taken into consideration.
Knowing the serotype distribution of GBS is vital to understand the epidemiology of GBS infections. Currently, a total of 10 distinct GBS capsular serotypes (Ia, Ib, and II–IX) have characterized according to the capsular polysaccharide (CPS), one of the major known virulence factors underlying invasive GBS disease [86]. The five serotypes such as Ia, Ib, II, III, and V are the most common which accounted for more than 85% of serotypes in the global regions that have reported serotype data, including the Americas (96%), Europe (93%), and the Western Pacific (89%) [87]. Correspondingly, in our meta-analysis, the five serotypes: V, III, Ia, Ib and II were the most common which accounted 91.8% among the 10 serotypes analyzed in the 21 African countries. In addition, the estimated mean prevalence of the serotypes Ia, Ib, or III reported in studies conducted in the USA and Europe were 55.0% (95% CI 52.3, 57.7), and 58.3% (95% CI 52.2, 64.5), respectively. It is slightly in agreement with our finding in which these three serotypes (Ia, Ib and III) accounted 62.4%. We also found that IX accounted 20.9% (95% CI 4.9, 37.0) compiled from four studies, and 7.0% (95% CI 3.4, 10.6) non-typeable (NT) GBS analyzed from the nine studies which could potentially be one of the problems for the development of effective maternal vaccine against GBS.
Limitation of the study
The availability of data on GBS serotype distribution in the African countries was limited, with nine studies included to this review. The finding of this study also showed certain heterogeneity although its appearance in the analysis of many studies conducted by different researchers is inevitable.
Conclusion
The data generated from this systematic review and meta-analysis provided important epidemiological information on colonizing GBS isolated from the 22,206 pregnant women in the 21 African countries. The most antibiotic resistance proportion estimate was observed in the tetracycline followed by penicillin which remains the drug of choice for GBS in the Westerns. Streptococcus agalactiae also exhibited considerable resistance to ampicillin and vancomycin which are usually recommended for maternal IAP in the developed countries. Serotype V, III, Ia, Ib, and II were found to be the most prevalent in the Africa that altogether accounted more than 91.8%. Findings of this review will contribute its part in the GBS vaccine development suited for disease prevention and treatment in Africa, as well as the implementation of effective clinical antibiotic usage. The authors recommended that infection and antibiotic resistance control strategies should include GBS as one of the most infective bacteria particularly for newborns delivered from the colonized pregnant women. In contrary to other continents, very few data are available in Africa about the GBS serotype profiles, thus, more data is needed in Africa to support the international community who are working on the GBS vaccine development.
References
Sambola A, Miro JM, Tornos MP, Almirante B, Moreno-Torrico A, Gurgui M, et al. Streptococcus agalactiae infective endocarditis: analysis of 30 cases and review of the literature, 1962–1998. Clin Infect Dis. 2002;34:1576–84.
Sørensen UBS, Poulsen K, Ghezzo C, Margarit I, Kilian M. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. mBio. 2010;1:e00178–210.
Centers for Disease Control and Prevention. Prevention of perinatal Group B Streptococcal disease: revised guidelines from CDC. MMWR Recomm Rep. 2010;59(No. RR-10):1–36.
Richards VP, Lang P, Bitar PD, Lefébure T, Schukken YH, Zadoks RN, et al. Comparative genomics and the role of lateral gene transfer in the evolution of bovine adapted Streptococcus agalactiae. Infect Genet Evol. 2011;11:1263–75.
Pereira UDP, dos Santos AR, Hassan SS, Aburjaile FF, Soares SDC, Ramos RTJ, et al. Complete genome sequence of Streptococcus agalactiae strain SA20-06, a fish pathogen associated to meningoencephalitis outbreaks. Standards Genom Sci. 2013;8:188–97.
Baker CJBF, Gordon RC, Yow MD. Suppurative meningitis due to Streptococci of Lancefield Group B: a study of 33 infants. J Pediatr. 1973;82(4):724–9.
Fluegge K, Siedle A, Heinrich B, Schulte-Moenting J, Moennig MJ, Bartels DBEA. Incidence and clinical presentation of invasive neonatal Group B Streptococcal infections in Germany. Pediatrics. 2006;117(6):139–45.
Schuchat A. Epidemiology of Group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513.
Hood M, Janney A, Dameron G. Beta hemolytic Streptococcus Group B associated with problems of the perinatal period. Am J Obstet Gynecol. 1961;82:809–18.
Woldu ZL, Teklehaimanot TG, Waji ST, Gebremariam MG. The prevalence of Group B Streptococcus recto-vaginal colonization and antimicrobial susceptibility pattern in pregnant mothers at two hospitals of Addis Ababa, Ethiopia. Reprod Health. 2014;11:80.
Mengist HM, Zewdie O, Belew A, Dabsu R. Prevalence and drug susceptibility pattern of Group B Streptococci (GBS) among pregnant women attending antenatal care (ANC) in Nekemte Referral Hospital (NRH), Nekemte, Ethiopia. BMC Res Notes. 2017;10:338.
Mohammed M, Asrat D, Woldeamanuel Y, Demissie A. Prevalence of Group B Streptococcus colonization among pregnant women attending antenatal clinic of Hawassa Health Center, Hawassa, Ethiopia. Ethiop J Health Dev. 2012;26(1):36–42.
Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal Group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res Notes. 2016;9:351.
Gebremeskel TK, Zeleke TA, Mihret A, Tikue MD. Prevalence and antibiotic susceptibility pattern of Streptococcus agalactiae among pregnant women at Adigrat Zonal Hospital and Adigrat Health Center, Tigray, Ethiopia. J Gynecol Obstet. 2015;3(2):29–35.
Assefa A, Desta K, Lema T. Group B streptococci vaginal colonization and drug susceptibility pattern among pregnant women attending in selected public antenatal care centers in Addis Ababa, Ethiopia. BMC Pregnancy Childbirth. 2018;18:135.
Yadeta TA, Worku A, Egata G, Seyoum B, Dadi M, Berhane Y. Vertical transmission of Group B Streptococcus and associated factors among pregnant women: a cross-sectional study, Eastern Ethiopia. Infect Drug Resist. 2018;11:397–404.
Alemseged A, Niguse S, Hailekiros H, Abdulkadir M, Saravanan M, Asmelash T. Isolation and anti-microbial susceptibility pattern of Group B Streptococcus among pregnant women attending antenatal clinics in Ayder Referral Hospital and Mekelle Health Center, Mekelle, Northern Ethiopia. BMC Res Notes. 2015;8:518.
Joachim A, Matee MI, Massawe FA, Lyamuya EF. Maternal and neonatal colonisation of Group B Streptococcus at Muhimbili National Hospital in Dar es Salaam, Tanzania: prevalence, risk factors and antimicrobial resistance. BMC Public Health. 2009;9:437.
Namugongo A, Bazira J, Fajardot Y, Joseph N. Group B Streptococcus colonization among pregnant women attending antenatal care at Tertiary Hospital in Rural Southwestern Uganda. Int J Microbiol. 2016;2016:7.
Kaminja C, Gichuhi JW, Kizito L. Maternal Group B Streptococcus colonization and the associated early maternal and neonatal outcomes at the Kisii teaching and referral hospital. Unpublished thesis 2011; H58/68573/2011:1–29.
Ernest A, Ng’Walida N, Ndaboine E, Massinde A, Kihunrwa A, Mshana S. Maternal vaginorectal colonization by Group B Streptococcus and Listeria monocytogenes and its risk factors among pregnant women attending tertiary hospital in Mwanza, Tanzania. Tanzania J Health Res. 2015;17(2):1–7.
Schmidt J, Halle E, Halle H, Mohammed T, Gunther E. Colonization of pregnant women and their newborn infants with Group B Streptococci in the Gondar College of Medical Sciences. Ethiop Med J. 1989;27:115–9.
Gizachew M, Tiruneh M, Moges F, Adefris M, Tigabu Z, Tessema B. Streptococcus agalactiae from Ethiopian pregnant women; prevalence, associated factors and antimicrobial resistance: alarming for prophylaxis. Ann Clin Microbiol Antimicrob. 2019;18(1):3.
Musa M, Woldeamanuel Y, Asrat D. Group B Streptococci: colonization rate among pregnant women and their newborn and burden of neonatal disease in selected hospitals of Ethiopia. AAU Institutional Repository (PhD Dissertation, unpublished data). 2018. p. 1–228.
Elhassen AAEMA, Hamedelnil YF. Frequency of Group B Streptococcus agalactiae (GBS) Among sudanese pregnant women with previous miscarriage in Khartoum. Sudan University of Science and Technology College of Graduate Studies (M.Sc. Thesis, unpublished data). 2018. p. 1–51.
Salano JC, McClelland S. Prevalence, antimicrobial susceptibility and serotypes of Group B Streptococcus rectovaginal isolations from pregnant women at Kenyatta National Hospital W64/76432/2014 (M.Sc. Thesis, unpublished data). 2018. p. 1–87.
Mitima KT, Ntamako S, Birindwa AM, Mukanire N, Kivukuto JM, Tsongo K, et al. Prevalence of colonization by Streptococcus agalactiae among pregnant women in Bukavu, Democratic Republic of the Congo. Infect Dev Ctries. 2014;8(9):1195–200.
Belard S, Toepfner N, Capan-Melser M, Mombo-Ngoma G, Zoleko-Manego R, Groger M, et al. Streptococcus agalactiae serotype distribution and antimicrobial susceptibility in pregnant women in Gabon, Central Africa. Sci Rep. 2015;2:17281.
Edmond T, Malick ZF, Yehouenou LC, Bankolé HS, Wilfried BK, Marius EA, et al. Bacterial distribution and antibiotic susceptibility pattern of Group B Streptococcus β hemolytic (GBS) in vaginal infections at Cotonou in Benin. Am J Infect Dis Microbiol. 2017;5(3):109–14.
Vinnemeier CD, Brust P, Owusu-Dabo E, Sarpong N, Sarfo EY, Bio Y, et al. Group B Streptococci serotype distribution in pregnant women in Ghana: assessment of potential coverage through future vaccines. Trop Med Int Health. 2015;20(11):1516–24.
Banini JA, Frimpong EH. Carriage and antibiotic susceptibility profile of Group B Streptococcus during late pregnancy in selected hospitals in greater Accra. Unpublished Thesis. 2014; PG6068111. p. 1–54.
Enweronu-Laryea CC, Damale NRK, Newman MJ. Prevalence of Group B Streptococcus in pregnant women attending a tertiary hospital in Ghana in 2001. Archiv Clin Microbiol. 2001;2(2):5.
Le Doare K, Jarju S, Darboe S, Warburton F, Gorringe A, Heath PT, et al. Risk factors for Group B Streptococcus colonisation and disease in Gambian women and their infants. J Infect. 2016;72(3):283–94.
Roca A, Bojang A, Camara B, Oluwalana C, Lette K, West P, et al. Maternal colonization with Staphylococcus aureus and Group B Streptococcus is associated with colonization in newborns. Clin Microbiol Infect. 2017;23:974–9.
Ezeonu IM, Agbo MC. Incidence and anti-microbial resistance profile of Group B Streptococcus (GBS) infection in pregnant women in Nsukka, Enugu State, Nigeria. Afr J Microbiol Res. 2014;8(1):91–5.
Onipede A, Adefusi O, Adeyemi A, Adejuyigbe E, Oyelese A, Ogunniyi T. Group B Streptococcus carriage during late pregnancy in Ile-Ife, Nigeria. Afr J Clin Exp Microbiol. 2012;13(3):135–43.
Akinniyi AM, Adesiyun AG, Kolawole A, Giwa F, Randawa A. The prevalence of asymptomatic Group B streptococcal infection and antimicrobial sensitivity pattern among parturients at Ahmadu Bello University Teaching Hospital, Zaria, Nigeria. Trop J Obstet Gynaecol. 2017;34:182–7.
Oob D, Okonko IO, Donbraye E, Fadeyi A, Abubakar MJ, Adebiyi OE, et al. Isolation and characterization of Group B Streptococci and other pathogens among pregnant women in Ibadan, Southwestern Nigeria. J Appl Biosci. 2010;29:1781–92.
Elikwu CJ, Oduyebo O, Ogunsola FT, Anorlu RI, Okoromah CN, König B. High Group B streptococcus carriage rates in pregnant women in a tertiary institution in Nigeria. Pan Afr Med J. 2016;25:249.
Ahidjo PE, Yang SE, Achidi E, Musonge B, Inoni L. Vaginal colonization and resistance profile of Group B Streptococcus among pregnant women in Yaoundé Gynecology, Obstetric and Pediatric Hospital in Cameroon. Afr J Immunol Res. 2015;2(1):071–5.
Mounerou S, Anoumou YD, Biova AA, Koumavi E, Sika D, Kpatcha K, et al. Group B streptococcal carriage rate in vagina of pregnant women in third trimester in Lomé, Togo. World J Prev Med. 2015;3(1):7–10.
Suara RO, Adegbola RA, Baker CJ, Secka O, Mulholland EK, Greenwood BM. Carriage of Group B Streptococci in pregnant Gambian mothers and their infants. J Infect Dis. 1994;170:1316–9.
Akinlolu JT, Omololu-Aso J, Owolabi AT, Omololu-Aso OO. Molecular epidemiological status of Group B Streptococcus in Ile Ife South Western Nigeria. Arch Med. 2018;10(3):4.
Biobaku OR, Olaleye AO, Adefusi OF, Adeyemi BA, Onipede AO, Loto OM, et al. Group B streptococcus colonization and HIV in pregnancy: a cohort study in Nigeria. J Neonatal Perinatal Med. 2017;10(1):91–7.
Njoku C, Emechebe C, Agbakwuru A. prevalence and determinants of anogenital colonization by Group B Streptococcus infection among HIV positive and negative women in Calabar, Nigeria. Int J Women’s Health Reprod Sci. 2017;6(1):11–7.
Anosike IK, Ebana RUB, Edet UO, Egbomuche RC, Victory AR. Prevalence of Streptococcus agalactiae among women resident in Calabar, Cross River State, Nigeria. Asian J Res Med Pharm Sci. 2017;2(2):1–7.
Nkembe NM, Kamga HG, Baiye WA, Chafa AB, Njotang PN. Streptococcus agalactiae prevalence and antimicrobial susceptibility pattern in vaginal and anorectal swabs of pregnant women at a tertiary hospital in Cameroon. BMC Res Notes. 2018;11:480.
Medugu N, Iregbu KC, Parker RE, Plemmons J, Singh P, Audu LI, et al. Group B streptococcal colonization and transmission dynamics in pregnant women and their newborns in Nigeria: implications for prevention strategies. Clin Microbiol Infect. 2017;23:673.e9–16.
Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of Group B Streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS ONE. 2011;6(3):e17861.
Mavenyengwa RT, Afset JE, Schei B, Berg S, Caspersen T, Bergseng H, et al. Group B Streptococcus colonization during pregnancy and maternal-fetal transmission in Zimbabwe. Acta Obstet Et Gynecol. 2010;89:250–5.
Mavenyengwa RT, Moyo SR, Nordbø SA. Streptococcus agalactiae colonization and correlation with HIV-1 and HBV seroprevalence in pregnant women from Zimbabwe. Eur J Obstet Gynecol Reprod Biol. 2010;150:34–8.
Dzowela T, Komolafa O, Igbigbi A. Prevalence of Group B Streptococcus colonization in ante-natal women at the Queen Elizabeth Central Hospital, Blantyre—a preliminary study. Malawi Med J. 2005;17(3):97–9.
Lekala LM, Mavenyengwa RT, Moyo SR, Lebelo SL, Bolukaoto JY, Chukwu MO, et al. Risk factors associated with Group B Streptococcus colonization and their effect on pregnancy outcome. J Gynecol Obstet. 2015;3(6):121–8.
Gray KJ, Kafulafula G, Matemba M, Kamdolozi M, Membe G, French N. Group B Streptococcus and HIV infection in pregnant women, Malawi, 2008–2010. Emerg Infect Dis. 2011;17(10):1932–5.
de Steenwinkel FDO, Tak HV, Muller AE, Nouwen JL, Oostvogel PM, Mocumbi SM. Low carriage rate of Group B Streptococcus in pregnant women in Maputo, Mozambique. Trop Med Int Health. 2008;13(3):427–9.
Monyama MC, Bolukaoto JY, Chukwu MO, Maloba MRB, Moyo SR, Mavenyengwa RT, et al. Group B Streptococcus colonisation in pregnant women at Dr. George Mukhari Hospital, South Africa. Southern Afr J Infect Dis. 2016. https://doi.org/10.1080/23120053.2016.1156308.
Bolukaoto JY, Monyama CM, Chukwu MO, Lekala SM, Nchabeleng M, Maloba MRB, et al. Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC Res Notes. 2015;8:364.
Mason PR, Gwanzura L, Latif AS, Ray S, van de Wijgert J, Katzenstein DA. Antimicrobial susceptibility patterns amongst Group B Streptococci from women in Harare, Zimbabwe. Int J Antimicrob Agents. 1996;7:29–32.
Africa CWJ, Kaambo E. Group B Streptococcus serotypes in pregnant women from the Western Cape Region of South Africa. Front Public Health. 2018;6:356.
Madrid L, Maculuve SA, Vilajeliu A, Sáez E, Massora S, Cossa A, et al. Maternal carriage of Group B Streptococcus and Escherichia coli in a District Hospital in Mozambique. Pediatr Infect Dis J. 2018;37(11):1145–53.
Engelbrecht F, Moyo SR, Maposa I, Mukesi M, Khan S. The antimicrobial susceptibility and gene based resistance of Streptococcus agalactiae (Group B Streptococcus), in pregnant women in Windhoek (Khomas region), Namibia. Med Technol SA. 2017;30:9–14.
Sadaka SM, Aly HA, Meheissen MA, Orief YI, Arafa BM. Group B streptococcal carriage, antimicrobial susceptibility, and virulence related genes among pregnant women in Alexandria, Egypt. Alexandria J Med. 2018;54:69–76.
Ferjani A, Abdallah H, Saida N, Gozzi C, Boukadida J. Vaginal colonization of the Streptococcus agalactiae in pregnant woman in Tunisia: risk factors and susceptibility of isolates to antibiotics. Bull Soc Pathol Exot. 2006;99(2):99–102.
Shabayeka SAAE, Abdallaa SM, Abouzeidb AMH. Vaginal carriage and antibiotic susceptibility profile of Group B Streptococcus during late pregnancy in Ismailia, Egypt. J Infect Public Health. 2009;2:86–90.
Shabayek S, Abdalla S. Macrolide- and tetracycline-resistance determinants of colonizing Group B Streptococcus in women in Egypt. J Med Microbiol. 2014;63:1324–7.
Moraleda C, Benmessaoud R, Esteban J, López Y, Alami H, Barkat A, et al. Prevalence, antimicrobial resistance and serotype distribution of Group B Streptococcus isolated among pregnant women and newborns in Rabat, Morocco. J Med Microbiol. 2018. https://doi.org/10.1099/jmm.0.000720.
Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, et al. Prevalence of maternal colonisation with Group B Streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1076–84.
Barcaite E, Bartusevicius A, Tameliene R, Kliucinskas M, Maleckiene L, Nadisauskiene R. Prevalence of maternal Group B streptococcal colonization in European countries. Acta Obstet Gynecol Scand. 2008;87(3):260–71.
Russell NJ, Seale A, O’Driscoll M, O’Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, et al. Maternal colonization with Group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. CID. 2017;65(Suppl 2):S100–11.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6(7):1000097.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–8.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29.
Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–29.
Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5.
Borchardt SM, DeBusscher JH, Tallman PA, Manning SDMC, Kurzynski TA, Foxman B. Frequency of antimicrobial resistance among invasive and colonizing Group B Streptococcal isolates. BMC Infect Dis. 2006;6:57.
Chohan L, Hollier LM, Bishop K, Kilpatrick CC. Patterns of antibiotic resistance among Group B Streptococcus isolates: 2001–2004. Infect Dis Obstet Gynecol. 2006;2006:57492.
Castor ML, Whitney CG, Como-Sabetti K. Antibiotic resistance patterns in invasive Group B streptococcal isolates. Infect Dis Obstet Gynecol. 2008;2008:727505.
Panda B, Iruretagoyena I, Stiller R, Panda A. Antibiotic resistance and penicillin tolerance in ano-vaginal Group B Streptococci. J Matern Fetal Neonatal Med. 2009;22:111–4.
Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, et al. First molecular characterization of Group B Streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemoth. 2008;52:2890–7.
Dahesh S, Hensler ME, Van Sorge NM, Gertz RE Jr, Schrag S, Nizet V, et al. Point mutation in the Group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother. 2008;52:2915–8.
Metcalf BJ, Chochua S, Gertz RE, Hawkins PA, Ricaldi J, Li Z, et al. Active Bacterial Core surveillance team Short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiae recovered in the USA. Clin Microbiol Infect. 2017;23(8):574.e7–14.
Park C, Nichols M, Schrag SJ. Two cases of invasive vancomycin-resistant Group B Streptococcus infection. N Engl J Med. 2014;370(9):885–6.
Yu H, Lin H, Yang P, Hsu C, Hsieh W, Tsao L, et al. Group B streptococcal infection in Taiwan: maternal colonization and neonatal infection. Pediatr Neonatol. 2011;52:190–5.
Melin P, Efstratiou A. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine. 2013;31(Suppl 4):D31–42.
Authors’ contributions
MG; Conceptualization; MG, MT, FM, BT: data creation; MG, MT: Formal analysis; MG, MT, FM, BT: Investigation; MG, FM, BT: Methodology; MG: Project administration; MG: Software; BT, MT, FM: Supervision; MG, MT, FM, BT: Validation; BT: Visualization; MG: writing the original draft; MG, MT, FM, BT; writing the review and editing. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge the authors of each study, and the colleagues (Muluget Melku, and Zegeye Abebe) who supported to reach this manuscript at this level.
Competing interests
The authors declare that they have no competing interest.
Availability of data and materials
Not applicable to data. All information utilized could be found using the references provided in body of the manuscript/article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding source
There was no funding source for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gizachew, M., Tiruneh, M., Moges, F. et al. Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: a meta-analysis. Ann Clin Microbiol Antimicrob 18, 14 (2019). https://doi.org/10.1186/s12941-019-0313-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12941-019-0313-1