Abstract
Background
The effects of alpha-linolenic acid (ALA) on cardiovascular risk factors considerably vary between published reports. Therefore, we investigated the effects of 12-week supplementation with flaxseed oil (FO), which is a rich source of ALA, on cardiovascular risk factors such as serum small dense low-density lipoprotein (sd-LDL) concentrations.
Methods
In a randomized, double blind, crossover study, 15 subjects ingested 10 g of FO or corn oil (CO), containing 5.49 g and 0.09 g of ALA, respectively, once daily with dinner. Blood samples were collected at 0, 4 and 12 weeks, and were used for analysis of serum lipid, lipid-related proteins, serum fatty acids and serum sd-LDL cholesterol. Differences during the test period were identified using a repeated-measures analysis of variance (ANOVA) for within-group effects. Group differences were identified using paired t-test at each blood sampling time point.
Results
ALA and eicosapentaenoic acid concentrations were significantly higher in the FO period at 4 and 12 weeks than in the CO period. No significant differences in docosahexaenoic acid concentrations were observed between two periods, and cholesteryl ester transfer protein and apolipoprotein B concentrations were significantly lower in the FO period than in the CO period at 12 weeks. FO supplementation was associated with a significant decrease in sd-LDL concentrations at 4 and 12 weeks, and CO supplementation had no effect. Moreover, sd-LDL concentrations were significantly lower in the FO period than in the CO period at 4 weeks. Among subjects with triglyceride (TG) concentrations of >100 mg/dl, FO supplementation markedly reduced sd-LDL concentrations at 4 and 12 weeks compared with baseline. Sd-LDL concentrations significantly differed between the periods at both 4 and 12 weeks.
Conclusion
This study indicates that the FO, which is a rich source of ALA, leads to lower sd-LDL cholesterol concentrations.
Similar content being viewed by others
Background
Metabolic syndrome is a cluster of inter-related plasma lipid and lipoprotein abnormalities, including reduced high-density lipoprotein (HDL) cholesterol, a predominance of small dense low-density lipoprotein (sd-LDL) particles, and increased triglyceride (TG) concentration [1]. These dyslipidemic features are associated with increased risk of cardiovascular disease (CVD) [2].
Subclasses of LDL are characterised by variations in density, size and chemical composition and are of clinical importance [3]. Sd-LDL is a risk factor for the development of coronary artery disease in Westerners [4,5] and Japanese with relatively low LDL cholesterol concentrations [6,7]. In a recent study, sd-LDL cholesterol was significantly associated with CVD development in a Japanese population [8], indicating the importance of sd-LDL cholesterol as a biomarker to predict CVD.
Numerous studies have investigated the influence of n-3 fatty acids on CVD risk, and most of the health benefits observed in these studies have been attributed to the seafood n-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [9,10]. Compared with seafood n-3 fatty acid, fewer studies have evaluated the relationship between plant-derived n-3 fatty acid, alpha-linolenic acid (ALA), and the risk of coronary heart disease and other CVD outcomes. Previous meta-analysis showed that consumption of ALA might reduce heart disease mortality [11], whereas other previous study showed no significant cardiovascular benefit of ALA supplementation [12,13]. Thus, the effects of ALA have been inconsistent, and previous systematic review pointed out some methodologic problems [12]. However, recently there has been a steady increase in the number of studies supporting specific health benefits of ALA [14,15]. Furthermore, because seafood n-3 fatty acids consumption is limited by availability, plant-based n-3 fatty acids containing ALA, which can be elongated and desaturated to EPA and DHA [16], may be an important dietary alternative source for the maintenance of optimal EPA and DHA concentrations in plasma and cell membranes. Therefore, characterization of the cardioprotective effects of ALA is of considerable importance with regard to public health.
Therefore, the objective of this study was to elucidate the effects of 12-week supplementation with flaxseed oil (FO), which is a rich source of ALA, on cardiovascular risk factors, including sd-LDL cholesterol.
Methods
Subjects
Twenty-six adult males with healthy and social lifestyles were screened. Potential participants were screened by medical history, physical examination, laboratory analysis, and daily intake of fish. Subjects with evidence of infections, diabetes mellitus, renal, liver or inflammatory disease were excluded. Subjects who regularly consumed FO or perilla oil were also excluded. Among twenty-one enrolled men, 1 withdrew before completion of the study due to medication and 5 subjects were excluded for non-compliance during the 12-week intervention. From these participants, 15 subjects with a mean ± standard error (SE) body mass index (BMI, kg/m2) of 25.1 ± 0.5 and a mean ± SE age of 44.5 ± 3.1 y completed the study. Four subjects were smokers and 5 had received anti-hypertensive drugs. Subject characteristics and laboratory data are presented in Table 1. According to TG concentrations, 15 subjects were divided into two groups: the TG < 100 mg/dl group (n = 5), and the TG > 100 mg/dl group (n = 10).
Informed consent was obtained from all participants before the start of the study, which was approved by the ethical committee of Tokushima University Hospital, Tokushima, Japan. The study was performed in accordance with the Helsinki Declaration.
Study design
In a randomized, double blind, crossover study, subjects were given either FO or corn oil (CO) during two consecutive 12-week supplementation periods. The supplementation periods were separated by a 8-week wash-out period. The subjects were instructed to ingest 10 g of FO or CO using the spoon provided, once daily with dinner. Supplements of 10 g of FO and CO contained 5.49 and 0.09 g of ALA, respectively. Fatty acid compositions of the two oils are presented in Table 2. The fatty acid composition of the two oils was measured by gas–liquid chromatography. To avoid oxidation, they were instructed to put oil bottles in a box for blocking out light and to store in the refrigerator.
Subjects were instructed to maintain their habitual diet throughout the study to exclude influences of dietary nutrients, and were asked to continue with their normal daily activities. Subjects were also instructed to avoid intake of anti-inflammatory drugs, vitamins or other dietary supplements throughout the intervention period.
Subjects recorded their daily activities and food intake in a diary, and these life-style records were confirmed during interviews every 2 weeks. Subjects returned used oil bottles and were provided with oil supplements for the following 2 weeks. Remaining volumes in returned bottles were measured to assess compliance. All subjects consumed more than 97% of the assigned oil quantity.
Subjects were visited at 0 (run-in period), 4 and 12 weeks to collect fasting blood samples, body weight, and blood pressure measurement, and to hand in their previous 3-day’s dietary records. Subjects were instructed to eat and drink the same prescribed foods at 20.00 h prior to scheduled visits. Fasting blood samples were collected at 08.00 h after overnight fasting, and were used for analysis of serum lipid, lipid-related proteins, serum fatty acids and serum sd-LDL cholesterol. A dietician calculated mean energy intake from each subject’s dietary records of the 3 days leading up to scheduled visits. These dietary records were analyzed using computerized software (Excel Eiyou-kun version 4.0, Kenpaku-sha, Tokyo, Japan) to determine caloric intake and macronutrient content.
Analytic methods
Blood sample were centrifuged at 3000 rpm for 10 min at 4°C and then separated into plasma or serum. Alpha-tocopherol was measured using high-performance liquid chromatography. Total cholesterol was determined by the cholesterol dehydrogenase (UV-End) method. LDL-cholesterol and HDL-cholesterol were determined by a direct method. We calculated non-HDL cholesterol as the difference between total and HDL cholesterol. TG was determined by an enzyme method. The remnant like particles (RLP) -cholesterol was determined by the immune adherence method. Cholesterol ester transfer protein activity (CETP) was measured using a commercially available ELISA kit (Daiichi-kagaku, Tokyo Japan). Apolipoproteins (apo A-1, B, C-3 and E) were measured by the turbidimetric immunoassay (TIA) system. Apo B-48 was measured by the chemiluminescent enzyme immunoassay (CLEIA). Serum fatty acids composition was measured by gas–liquid chromatography. Briefly, total lipids in the serum were extracted using the Folch procedure and fatty acids were then methylated with BF3/methanol. Transesterified fatty acids was then analyzed using a gas chromatograph (GC-17A; Shimadzu, Kyoto, Japan) with a capillary column Omegawax 250 (Supelco, Bellefonte, PA). Measurements of sd-LDL-cholesterol were performed using sd-LDL-EX “SEIKEN” (Denka Seiken, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± SE. Baseline physical characteristic and laboratory data between the TG > 100 mg/dl group and the TG < 100 mg/dl group were compared using Mann–Whitney U test. Differences during the test period were identified using a repeated-measures analysis of variance (ANOVA) and the Bonferroni method for calculating 95% confidence interval (CI) after correcting for multiple comparisons for within-group effects. Group differences were identified using paired t-test at each blood sampling time point. Differences were considered significant when P < 0.05. Statistical analyzes were performed using SPSS for Windows, release 19.0 (SPSS, Chicago, IL).
Results
Food and nutrient intake
Dietary intake in CO and FO periods is shown in Table 3. At 0 week, no significant differences in total energy, protein, carbohydrate, fat, dietary fiber or alcohol intake were observed between the periods.
In the FO period, total ALA intake was significantly higher at 4 and 12 weeks than at 0 week. Total ALA intake at 4 and 12 weeks was significantly higher in the FO period than in the CO period. In the CO period, total linoleic acid (LA) intake was significantly higher at 4 and 12 weeks than at 0 week. Total LA intake at 12 weeks was significantly lower in the FO period than in the CO period.
In the FO period, ratios of n-6/n-3 were significantly lower at 4 and 12 weeks than at 0 week. In the CO period, ratios of n-6/n-3 were significantly higher at 4 and 12 weeks than at 0 week. Ratios of n-6/n-3 at 4 and 12 weeks were significantly lower in the FO period than in the CO period.
Alpha-tocopherol intakes at 12 weeks were significantly lower in the FO period than in the CO period.
Anthropometric and laboratory data
Anthropometric and laboratory parameters from subjects of CO and FO periods are shown in Table 4. No significant differences in body weight were observed at 0 week, and no significant differences in alpha-tocopherol concentrations were observed between the two periods at the completion of the study.
Changes in serum lipids and lipid-related proteins in CO and FO periods are shown in Table 5. No significant differences in lipids and lipid-related proteins were observed at 0 week. In the FO period, CETP concentrations were significantly lower at 12 weeks than at 0 week and were significantly lower than in the CO period at 12 weeks. Total cholesterol, LDL-, HDL-, non HDL-cholesterol, apolipoprotein (Apo) A-1 and Apo B concentrations were significantly lower in the FO period than in the CO period after 12 weeks. TG, RLP-cholesterol, apo C-3, apo E and apo B48 concentrations didn’t change significantly during the CO and FO periods.
Adverse events such as headache, fatigue, diarrhoea and stomach fullness were observed in both periods but were unrelated to the test diet. Moreover, no symptoms or side-effects of test diets were noted during inquiries about symptoms or during examinations.
Changes in serum fatty acid concentrations
Changes in serum fatty acid concentrations in CO and FO periods are shown in Table 6. With the exception of docosatetraenoic acid, no significant differences in serum fatty acid composition were detected between the two periods at 0 week. At 4 weeks, LA concentrations were significantly increased in the CO period compared with 0 week. Whereas in the FO period, concentrations of LA at 4 and 12 weeks were significantly lower than in the CO period. Arachidonic acid concentrations were significantly decreased at 12 weeks in both periods and were significantly lower in the FO period than in the CO period at 4 weeks.
ALA concentrations were significantly decreased at 12 weeks in the CO period, but were increased at 4 and 12 weeks in the FO period compared with 0 week and were significantly higher than in the CO period at these time points.
At 4 and 12 weeks, EPA concentrations were significantly higher in the FO period than in the CO period. However, no significant differences in docosapentaenoic acid or DHA concentrations were detected between the periods, and DHA concentrations significantly decreased between 0 week and 12 weeks in both periods. Ratios of n-6/n-3 significantly increased in the CO period at 12 weeks, but were significantly decreased in the FO period at 4 and 12 weeks compared with 0 week. Moreover, n-6/n-3 ratios significantly differed between the periods at 4 and 12 weeks.
Serum sd-LDL concentrations
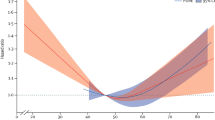
At 0 week, serum sd-LDL concentrations did not significantly differ between the two periods (data not shown). Changes in serum sd-LDL concentrations from 0 week are shown in Figure 1. FO supplementation was associated with 25.8% and 21.2% decreases in sd-LDL concentrations at 4 and 12 weeks, respectively, although CO supplementation did not affect sd-LDL concentrations (Figure 1A). At 4 weeks, sd-LDL concentrations were significantly lower in the FO period than in the CO period.
Mean (±SE) percentage changes from baseline in serum sd-LDL concentrations. 15 subjects (A), 10 subjects with TG concentrations of >100 mg/dl (B), 5 subjects with TG concentrations of <100 mg/dl (C); open circles indicate corn oil period, and closed circles indicate flaxseed oil period; *; p <0.05 vs. baseline, repeated-measures ANOVA with Bonferroni correction, †; p <0.05 vs. corn oil, paired t test.
Previous report showed that LDL particle size was influenced by TG concentrations [4]. To elucidate the effect of baseline TG concentrations, we divided subjects into two groups according to TG concentrations. Except for TG concentrations, there were no significant differences between the TG > 100 mg/dl group and the TG < 100 mg/dl group at baseline (Table 1), although sdLDL concentrations tended to be different (p = 0.055). Among subjects with TG concentrations of >100 mg/dl, FO supplementation led to 28.2% and 28.9% reductions in sd-LDL concentrations at 4 and 12 weeks compared with 0 week, respectively, and sd-LDL concentrations significantly differed between the periods at both 4 and 12 weeks (Figure 1B). However, among subjects with TG concentration of <100 mg/dl, sd-LDL concentrations did not differ between FO or CO supplementation at any time point, but were significantly lower at 4 weeks in the FO period compared with 0 week (Figure 1C).
Discussion
In the present study, we showed that FO supplementation markedly reduces serum sd-LDL concentrations, particularly in subjects with TG concentrations of >100 mg/dl. We also demonstrated that serum total cholesterol, LDL-cholesterol, HDL-cholesterol, CETP, Apo A-1 and Apo B concentrations were significantly lower in the FO period than in the CO period after 12 weeks.
FO used in this study is a rich source of ALA, and previous meta-analyzes suggest that ALA consumption may confer cardiovascular benefits, with a 10% decrease in risk of CHD mortality for each 1 g/d increment of ALA intake [14]. Furthermore, a recently published review showed the current evidence for an association between ALA and type 2 diabetes, and fracture risk, in addition to CVD outcomes [15].
ALA is converted in the liver into EPA and DHA [16,17], and n-6 fatty acids such as LA [18:2(n-6)] are believed to decrease the conversion of ALA to longer-chain n-3 PUFA by competing with ALA for binding to the rate-limiting enzyme Δ6-desaturase [16,18]. Other dietary factors such as polyunsaturated to saturated fat ratios, relative consumption of EPA and trans fatty acids and amounts and types of protein consumed have also been shown to affect this conversion [19]. In the present study, EPA concentrations were significantly higher after 4 and 12 weeks in the FO period than in the CO period, but DHA concentrations did not differ between the periods. Reportedly, humans convert <5% of dietary ALA to EPA or DHA [12,20]. In a previous randomised, double-blind trial, 56 participants were given 3-g doses of ALA/d from FO or olive oil placebo capsules. After 12 weeks, plasma EPA and docosapentaenoic acid (DPA) concentrations were significantly increased among subjects taking FO, whereas plasma DHA concentrations did not change [21]. Thus, the present increases in serum EPA concentrations may be clinically relevant. However, in agreement with previous studies [22,23], increased ALA intake failed to increase plasma phospholipid DHA concentrations, suggesting that the conversion to DHA is extremely low.
Previous study showed that non HDL-cholesterol (LDL + IDL + VLDL cholesterol) was more potent predictors of CVD [24]. In this study, non HDL-cholesterol was significantly lower in the FO period than in the CO period after 12 weeks. Therefore, it indicates that the FO supplementation could contribute to the improvement of the lipid profile. On the other hand, HDL-cholesterol concentrations were also significantly lower in the FO period than in the CO period in this study. Other studies also showed that HDL-cholesterol concentrations were lower in the ALA group than in the LA group [25,26]. In another study, no significant differences in HDL-cholesterol concentrations were found compared with the ALA group and the LA group [27]. Therefore, the effect of ALA on HDL-cholesterol concentrations is not consistent.
Increased hepatic production and/or retarded clearance from plasma of large VLDL result in increased production of precursors of sdLDL particles. Thus far, seven distinct LDL subspecies, which differ in their metabolic behavior and pathological rules, have been identified [28]. Plasma VLDL concentrations correlate with increased density and decreased sizes of LDLs [29,30]. In addition, LDL size and density are inversely correlated with plasma concentrations of HDLs, particularly those of the HDL2 subclass [31]. Sd-LDL particles are produced from intravascular processing of specific larger VLDL precursors through a series of steps, including lipolysis [28]. A common lipoprotein profile, designated the atherogenic lipoprotein phenotype, is characterised by a predominance of sd-LDL particles. Multiple features of this phenotype, including increased concentrations of triglyceride rich lipoprotein remnants and IDLs, and reduced concentrations of HDL and associated insulin resistance, contribute to the risk of coronary heart disease, particularly compared with individuals with predominantly larger LDLs. Reportedly, sd-LDL uptake by arterial tissue is greater than for larger LDLs [32], suggesting greater transendothelial transport of smaller particles. In addition, smaller LDL particles may have decreased receptor-mediated uptake and increased proteoglycan binding [33,34]. Several in vitro studies have demonstrated that LDL subfractions differ in their susceptibility to oxidative stress, which is a significant atherogenic factor [35,36]. Thus, increased concentrations of sd-LDL subfractions may significantly contribute to cardiovascular risk [37,38]. In the present study, sd-LDL concentrations were markedly reduced in the FO period, particularly in subjects with TG concentrations of >100 mg/dl. Thus, ALA in FO may lower sd-LDL cholesterols and contribute to reduce cardiovascular disease risk. Furthermore, although TG normal levels are defined as <150 mg/dl, previous study showed that the TG cutoff point that best distinguishes the two phenotypes, which are characterized by a predominance of large, buoyant LDL particles and sd-LDL particles, was 95 mg/dl in primarily healthy study sample [4]. Therefore, we considered that the changes of serum sd-LDL concentrations, particularly in subjects with TG concentrations of >100 mg/dl could be observed. However, we must take into account the small subgroup sample size in this study. Studying more subjects is clearly needed to determine the effect of TG concentrations on the FO supplementation.
Previously, it was reported that n-3 fatty acids, such as ALA, EPA and DHA, reduced secretion of apo B, induced degradation of apo B [39,40] and decreased hepatic VLDL production [39]. There are two types of apo B (apo B100 and apo B48). Apo B100 is produced in the liver and apo B48 is produced in the proximal small intestine. In this study, we analyzed apo B and apo B48 concentrations. Apo B concentrations were significantly lower in the FO period than in the CO period after 12 weeks, although apo B48 concentrations didn’t change significantly during the CO and FO periods. These results suggest that apo B, especially apo B100 concentrations, were significantly lower in the FO period. Furthermore, a previous report showed that degradation of CETP activity after EPA treatments may contribute to lower sd-LDL concentrations [41]. In this study, serum ALA and EPA concentrations were significantly increased in the FO period compared with the CO period, and CETP and Apo B concentrations were significantly lower in the FO period than in the CO period. These data suggest that FO-mediated decreases in sd-LDL concentrations may reflect decreases in apo B and VLDL concentrations. A limitation of our study is that we didn’t analyze the discrimination of HDL2/HDL3-subclasses. Previous report suggested that LDL size and density are inversely correlated with plasma concentrations of HDLs, particularly those of the HDL2 subclass [31]. Therefore, it is necessary to analyze the discrimination of HDL2/HDL3-subclasses for further study.
This study has shown that FO supplementation markedly decreases serum sd-LDL concentrations, particularly in subjects with TG concentrations of >100 mg/dl. In conclusion, this study indicates that the FO, which is a rich source of ALA, leads to lower sd-LDL cholesterol concentrations.
Abbreviations
- ALA:
-
Alpha-linolenic acid
- FO:
-
Flaxseed oil
- sd-LDL:
-
Small dense low-density lipoprotein
- CO:
-
Corn oil
- TG:
-
Triglyceride
- HDL:
-
High-density lipoprotein
- CVD:
-
Cardiovascular disease
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- CETP:
-
Cholesteryl ester transfer protein
- Apo:
-
Apolipoprotein
References
Haffner SM, Association AD. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26 Suppl 1:S83–6.
Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496–504.
Austin MA, Hokanson JE, Brunzell JD. Characterization of low-density lipoprotein subclasses: methodologic approaches and clinical relevance. Curr Opin Lipidol. 1994;5:395–403.
Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82:495–506.
Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–8.
Nozue T, Michishita I, Ishibashi Y, Ito S, Iwaki T, Mizuguchi I, et al. Small dense low-density lipoprotein cholesterol is a useful marker of metabolic syndrome in patients with coronary artery disease. J Atheroscler Thromb. 2007;14:202–7.
Koba S, Hirano T, Yoshino G, Sakai K, Sakaue T, Adachi M, et al. Remarkably high prevalence of small dense low-density lipoprotein in Japanese men with coronary artery disease, irrespective of the presence of diabetes. Atherosclerosis. 2002;160:249–56.
Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, et al. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb. 2013;20:195–203.
Kromhout D. Omega-3 fatty acids and coronary heart disease. The final verdict? Curr Opin Lipidol. 2012;23:554–9.
Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012;107 Suppl 2:S201–13.
Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134:919–22.
Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17.
Geleijnse JM, de Goede J, Brouwer IA. Alpha-linolenic acid: is it essential to cardiovascular health? Curr Atheroscler Rep. 2010;12:359–67.
Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, et al. α-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr. 2012;96:1262–73.
Rajaram S. Health benefits of plant-derived α-linolenic acid. Am J Clin Nutr. 2014;100:443S–8.
Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–9.
Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–76.
Emken EA, Adlof RO, Duval SM, Nelson GJ. Effect of dietary docosahexaenoic acid on desaturation and uptake in vivo of isotope-labeled oleic, linoleic, and linolenic acids by male subjects. Lipids. 1999;34:785–91.
Sugano M, Ikeda I. Metabolic interactions between essential and trans-fatty acids. Curr Opin Lipidol. 1996;7:38–42.
Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. 2002;5:127–32.
Harper CR, Edwards MJ, DeFilippis AP, DeFilipis AP, Jacobson TA. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr. 2006;136:83–7.
Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am J Clin Nutr. 2001;73:539–48.
Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, et al. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr. 1999;69:872–82.
Jiang R, Schulze MB, Li T, Rifai N, Stampfer MJ, Rimm EB, et al. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2004;27:1991–7.
Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet FA, Lefrandt JD, et al. Effect of an increased intake of alpha-linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean Alpha-linolenic Enriched Groningen Dietary Intervention (MARGARIN) study. Am J Clin Nutr. 2002;75:221–7.
Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–42.
Pang D, Allman-Farinelli MA, Wong T, Barnes R, Kingham KM. Replacement of linoleic acid with alpha-linolenic acid does not alter blood lipids in normolipidaemic men. Br J Nutr. 1998;80:163–7.
Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79.
McNamara JR, Jenner JL, Li Z, Wilson PW, Schaefer EJ. Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb. 1992;12:1284–90.
McNamara JR, Campos H, Ordovas JM, Peterson J, Wilson PW, Schaefer EJ. Effect of gender, age, and lipid status on low density lipoprotein subfraction distribution. Results from the Framingham Offspring Study. Arteriosclerosis. 1987;7:483–90.
Krauss RM, Williams PT, Lindgren FT, Wood PD. Coordinate changes in levels of human serum low and high density lipoprotein subclasses in healthy men. Arteriosclerosis. 1988;8:155–62.
Björnheden T, Babyi A, Bondjers G, Wiklund O. Accumulation of lipoprotein fractions and subfractions in the arterial wall, determined in an in vitro perfusion system. Atherosclerosis. 1996;123:43–56.
La Belle M, Krauss RM. Differences in carbohydrate content of low density lipoproteins associated with low density lipoprotein subclass patterns. J Lipid Res. 1990;31:1577–88.
Camejo G, López A, López F, Quiñones J. Interaction of low density lipoproteins with arterial proteoglycans. The role of charge and sialic acid content. Atherosclerosis. 1985;55:93–105.
Chait A, Brazg RL, Tribble DL, Krauss RM. Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med. 1993;94:350–6.
Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM. Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am J Med. 2001;110:103–10.
St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard PM, Després JP, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25:553–9.
Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–63.
Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, et al. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–63.
Pan M, Cederbaum AI, Zhang YL, Ginsberg HN, Williams KJ, Fisher EA. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J Clin Invest. 2004;113:1277–87.
Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30:144–6.
Acknowledgements
We are grateful to the volunteers who participated in the study. The present study was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries (MAFF) for a food research project titled ‘Integrated Research on Safety and Physiological Function of Food’ (to E. T. and H. Y.-O.) and SADAMITSU FOOD INDUSTRY LIMITED.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
YK analyzed and interpreted the data and wrote the manuscript. HY-O collected and analyzed the data, contributed to the design of the study and edited the manuscript. YN-K and MS collected, analyzed and interpreted the data. YT contributed significant advice. ET contributed significant advice and edited the manuscript. All the authors contributed to revisions of the manuscript and reviewed the final version.
Yuka Kawakami, Hisami Yamanaka-Okumura and Yuko Naniwa-Kuroki contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kawakami, Y., Yamanaka-Okumura, H., Naniwa-Kuroki, Y. et al. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: a randomized, double blind, crossover study. Nutr J 14, 39 (2015). https://doi.org/10.1186/s12937-015-0023-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-015-0023-2