Abstract
Background
Ovarian cancer is the fifth leading cause of cancer deaths in women worldwide. LncRNACCAT1 was reported to play a critical role in cell metastasis of ovarian cancer. However, little is known about the detailed mechanism of how CCAT1 enhances TGFβ1-induced EMT of ovarian cancer cells.
Methods
We used RT-qPCR to examine the level of miR-490-3p and CCAT1 and western blot to detect the protein level of TGFβR1 and EMT-associated markers. We utilized luciferase reporter assay to confirm the direct interaction of CCAT1 or TGFβ1 with miR-490-3p. Wound healing and invasion assay were employed to investigate the role of CCAT1 and miR-490-3p in the TGFβ1-induced migration and cell invasion of ovarian cancer cells, respectively.
Results
TGFβ1 stimulated the expression of CCAT1. And CCAT1 knockdown decreased cell migration, invasion and EMT-associated markers expression of ovarian cancer cells treated with TGFβ1. CCAT1 directly targeted and downregulated miR-490-3p, then increasing TGFβR1 level. miR-490-3p was shown to regulate cell invasion, migration and EMT markers expression via TGFβR1. In addition, we also observed that miR-490-3p was essential for TGFβ1-induced tumor cell invasion and migration influenced by CCAT1. CCAT1 level was significantly higher in tumors than adjacent normal tissue, in contrast, miR-490-3p level was lower in ovarian tumors.
Conclusion
Here, we reveal that CCAT1 contributes to TGFβ1-induced EMT of ovarian tumor cells through miR-490-3p/TGFR1 axis. These findings will provide deep insights into the mechanism by which CCAT1 exerts its oncogenic role in ovarian cancer progression and facilitate developing novel therapeutical therapies for treating ovarian cancer.
Similar content being viewed by others
Background
Ovarian cancer is one of the most lethal cancers and the fifth leading cause of cancer-associated death. However, little improvement of survival rate has been achieved over the past decade [1,2,3]. Patients diagnosed and treated with early stages have a 5-year survival rate over 90%. Unfortunately, the vast majority of ovarian cancer patients are diagnosed with advanced disease and 5-year survival is less than 30% [4]. Hence, the comprehensive understanding of the molecular mechanism of ovarian cancer metastasis is a key issue.
Epithelial–mesenchymal transition (EMT) is a developmental process whereby epithelial cells reprogram to a mesenchymal-like phenotype. Tumor cells undergo EMT change, a key prerequisite for metastasis, which can be initiated or controlled by various intracellular signaling pathway in response to environmental cues, including transforming growth factor beta1(TGFβ1) signaling [5, 6]. On one hand, TGFβ1 directly induces expression of EMT transcription factors, such as Snail, Slug, zinc finger E-box-binding homeobox1/2(ZEB1/2) and Twist, through Smad pathway [7, 8]. One the other hand, TGFβ1 promotes EMT via activation of PI3K/Akt/mTOR or mitogen-activated protein kinase (MAPK) pathway [9, 10]. Several studies suggest that the TGFβ1 is involved in ovarian cancer EMT progression. For example, it was report that TGFβ1 was upregulated in ovarian CAF-derived exosomes, which enhanced migration and invasion ability and the promotion of EMT by activating the SMAD signaling pathway [11]. Inhibitor of DNA binding 1 (Id-1), a protein repressed by miR-29b, facilitates the TGFβ1-induced EMT in human ovarian cancer cells [12]. However, little is known about the detailed mechanism of how TGFβ1 induces EMT of ovarian cancer cells.
Long ncRNAs, defined as a form of ncRNAs greater than 200 nt in length, are found to exert their gene transcription regulatory function by epigenetic regulatory mechanism [13,14,15]. Colon cancer-associated transcript 1 (CCAT1), ~ 2-kb lncRNA located at chromosome 8q24.21, is first found to be upregulated in colon cancer [16]. Recently, CCAT1 has been reported to be involved in a variety of cancers, including hepatocellular carcinoma [17], gallbladder cancer [18], gastric cancer [19] and colorectal cancer [20]. Yuan Cao et al. showed that CCAT1 downregulation inhibited epithelial ovarian cancer cell EMT, migration and invasion through targeting miR-152 and miR-130b [21]. However, whether CCAT1 is implicated in TGFβ1-induced EMT of ovarian tumor cells remains unclear. Based on the above facts, we sought to clarify the mechanism by which CCAT1 promoted TGFβ1-induced EMT of ovarian cancer cells.
Over the past decades, microRNAs have been considered to modulate their target genes expression by binding the 3′-UTR of targeted genes. Pathologically, microRNAs are involved in a wide range of cancer cell phenotypes, such as cell proliferation, survival, invasion and EMT [22, 23]. For examples, aberrant expression of miR-200 family is strongly associated with pathologic EMT [24]. MiR-451 regulates migration of glioma cells through AMPK and mTOR signaling [25]. In bladder cancer, miR-148a suppresses EMT by establishing links between ERBB3/AKT2/c-myc and DNMT1 [26]. Recently, several studies have showed that miR-490-3p has an inhibitory role in EMT of hepatocellular carcinoma and colorectal cancer cells [27, 28]. Intriguingly, miR-490-3p inhibits colorectal cancer metastasis by targeting TGFβR1, a TGFβ1 cognate receptor [29]. Moreover, it was report that lncRNACCAT1 regulated gastric cancer cell migration by targeting miR-490-3p [30]. Besides, MiR-490-3p plays a tumour suppressor role in epithelial ovarian cancer,and overexpression of miR-490-3p was reported to promote G1/S arrest and apoptosis, reduce cell proliferation and invasion of ovarian cancer cells [31]. It remains unknown about whether CCAT1 regulates TGFβ1-induced EMT of ovarian tumor cells through miR490-3p.
In this study, we highlight that knockdown CCAT1 represses TGFβ1-induced EMT of ovarian cancer cells through miR-490-3p/TGFβR1 axis. These findings will provide more understanding of how CCAT1 contributes to ovarian cancer metastasis, which helps develop novel targeted drugs for treating ovarian cancer.
Materials and methods
Cell culture and transfection
Ovarian cancer cells (SKOV3 and CaOV3) and 293T cell were purchased from ATCC and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone) supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin at 37 °C, 5% CO2. TGFβ1 was purchased from R and D systems and was used to induce EMT in SKOV3 and CaOV3 (10 ng/ml) cells for the indicated time periods.
The TGFβR1 cDNA was subcloned into pCDNA3.1 vector which was transfected into cells using lipofectamine 2000 according to the instruction. For miR-490-3p mimics or miR-490-3p inhibitor transfection, we used LipofectamineVR LTX with PlusTM Reagent (Life Technologies) to transfect them into cells. All siRNAs, miR-490-3p mimics and miR-490-3p inhibitor were synthesized by GenePharma. The sequences are as follows:
miR-490-3p mimics: (sense) 5′-CAACCUGGAGGACUCCAUGCUC-3′; (antisense) 5′-GCAUGGAGUCCUCCAGGUUGUU-3′;
miR-490-3p inhibitor: 5′-CAGCAUGGAGUCCUCCAGGUUG-3′.
Patients and samples
A cohort of 25 ovarian tumor tissues and adjacent normal ovarian tissue samples were obtained from patients aged 25–55 undergoing wedge biopsy of the ovaries or adnexectomy due to myoma or adenomyosis, between 2016.6 and 2017.5. No patients had received chemotherapy or radiotherapy prior to surgery. Consent from all patients were obtained. Ovarian cancer was validated by histological examination in all cases according to World Health Organization criteria. Ovarian cancer and normal ovarian tissue specimens excised surgically from patients were immediately snap-frozen and stored in liquid nitrogen until use. This experiment was approved by ethic committee of the 2nd Affiliated Hospital of Harbin Medical University, and the tissues were acquired with the consent of patients.
Plasmid transfection and lentivirus package
The short hairpin RNAs (shRNA CCAT1) were cloned into PLKO.1 vector. To make lentiviruses, the packaging vectors (pPAX2 and pVSVG) and PLKO. shRNAs were co-transfected into 293T cells. The supernatant was harvested at 48 h after transfection. For virus infection, the virus supernatant was added to medium at 1:5 ratio, after 24 h, 2 μg/ml puromycin was used to select the positive cells.
Wound healing assay
Migration of cells were measured by a wound healing assay in vitro. Briefly, 2 × 105 SKOV3 or CaOV3 cells were seeded onto 6-well plates, with either sh-CCAT1 or sh-NC, and incubated in appropriate complete culture medium for 16 h under normoxic conditions at 37 °C. The monolayer was scratched and incubated in medium without FBS for 24 h. The wound width was measured after 24 h. Three different locations were visualized and photographed under inverted microscope.
Invasion assay
Invasion assay was performed using chambers with 8.0-μm pore membranes (Millipore). Ovarian cancer cells (1 × 105 cells) were resuspended in 200 µl of FBS-free medium, and then seeded into the top chamber with Matrigel-coated membrane. Next, 500 µl medium with 10% FBS was added to the bottom chamber as a chemoattractant. After 48 h of incubation, the invaded cells were fixed, stained with 0.005% crystal violet, and counted under the inverted microscope.
Luciferase reporter assays
CCAT1 or TGFβR1 mutant was generated using site-directed mutagenesis. Then, the sequence of the CCAT1 or TGFβR1 was cloned into the firefly luciferase-expressing vector pGL3-luciferase plasmid. As for luciferase assay, the SKOV3 or CaOV3 cells were seeded for triplicates in 24-well plates at the day before transfection, and co-transfected with the CCAT1 or TGFβR1 reporter vector and miR-490-3p. Then, the cells were harvested and lysed, and the luciferase activities were assayed using the Dual-Luciferase Reporter System (Promega). Three independent experiments were performed.
Western blot
The cells were harvested and washed with PBS buffer, then lysed by 1 × SDS loading buffer. The lysates were boiled at 100 °C for 5 min. The samples were centrifuged at 10,000 rpm for 1 min. Around 50 μg of total proteins was loaded onto SDS-PAGE gel and resolved. After that, the proteins were transferred to PVDF membrane at 300 mA for 1.5 h. The membrane was blocked with 5% non-fat milk in 1× TBST for 1 h at room temperature, the membrane was then incubated with primary antibodies at 4 °C overnight. The following day, the membrane was washed with 1× TBST for three times, 5 min each time. The membrane was incubated with secondary antibodies at room temperature for 1 h. Finally, the membrane was incubated with ECL solution and then exposed. The following antibodies were used: anti-TGFβR1 (cell signaling technology, USA), anti-E-cadherin (cell signaling technology, USA), anti-N-cadherin (cell signaling technology, USA), anti-Claudin (cell signaling technology, USA), anti-β-actin (Proteintech, USA), anti-MMP9 (Abcam, USA), anti-GAPDH (Proteintech, USA).
RT-qPCR
We extracted the RNA using Trizol method. Cells were lysed by Trizol buffer and then add chloroform to the mixture. The sample was centrifuged at 12,000 rpm for 10 min and transferred to new EP tube, mixed with equivalent volume of isopropanol, next, the resultant was centrifuged at 12,000 rpm for 10 min. Removing the supernatant and add 75% ethanol to wash the pellet and centrifuge. Finally, discard the ethanol and dry the pellet, use 20–30 μl Rnase-free H2O to resolve the RNA.
For reverse transcription, about 1 μg of total RNA was used for reverse transcription according to manufacturer instruction (TAKARA PrimeScript Kit). The expression of miR-490-3p was quantified by TaqMan miRNA assays (Applied Biosystems, Foster City, CA, USA).
For real time PCR, we used SYBR as probe dye and detected the signal, the GAPDH and U6 were used as internal control. The following primers were used:
CCAT1-QPCR-F: 5′-GCAGGCAGAAAGCCGTATCT-3′
CCAT1-QPCR-R: 5′-TCCCAGGTCCTAGTCTGCTT-3′
miR-490-3p-QPCR-F: 5′-CGCAACCTGGAGGACTCC-3′
miR-490-3p-QPCR-R: 5′-CGGCCCAGTGTTCAGACTAC-3′
TGFβR1-QPCR-F: 5′-GTGACAGATGGGCTCTGCTT-3′
TGFβR1-QPCR-R: 5′-AGGGCCAGTAGTTGGAAGTT-3′
Claudin-QPCR-F: 5′-TTTACTCCTATGCCGGCGAC-3′
Claudin-QPCR-R: 5′-GAGGATGCCAACCACCATCA-3′
E-cadherin-QPCR-F: 5′-TCACATCCTACACTGCCCAG-3;
E-cadherin-QPCR-R: 5′-AGTGTCCCTGTTCCAGTAGC-3′,
N-cadherin-QPCR-F: 5′-AGGGGACCTTTTCCTCAAGA-3′;
N-cadherin-QPCR-R: 5′-TCAAATGAAACCGGGCTATC-3′,
Vimentin-QPCR-F: 5′-GGACCAGCTAACCAACGACA-3′;
Vimentin-QPCR-R: 5′-AAGGTCAAGACGTGCCAGAG-3′,
MMP9-QPCR-F: 5′-TTCCAAACCTTTGAGGGCGA-3′;
MMP9-QPCR-R:5′-CTGTACACGCGAGTGAAGGT-3′,
GAPDH-QPCR-F: 5′-AGCCCAAGATGCCCTTCAGT-3′;
GAPDH-QPCR-R: 5′-AGCCCAAGATGCCCTTCAGT-3′,
U6-QPCR-F: 5′-CTCGCTTCGGCAGCACA-3′;
U6-QPCR-R: 5′-AACGCTTCACGAATTTGCGT-3′.
Statistical analysis
Each experiment was performed for three times, all values were presented as mean ± SD, comparison of two groups were performed using the two-tailed unpaired student’s t-test. One-way ANOVA was used for comparison among multiple groups and multiple comparisons were further performed using post hoc Turkey test. *P < 0.05 were considered statistically significant (*P < 0.05, **P < 0.01, and ***P < 0.001).
Results
LncRNA CCAT1 depletion attenuates TGFβ1-induced EMT of ovarian cancer cells
To characterize the role of CCAT1 in TGFβ1-induced EMT of ovarian cancer cells, we first determined the expression level of CCAT1 in SKOV3 and CaOV3 cells when treated with 10 ng/ml TGFβ1 for 48 h. The result showed CCAT1 was upregulated by TGFβ1 (Fig. 1a). Next, we generated stable CCAT1-depleted SKOV3 and CaOV3 cells by shRNAs approach (Fig. 1b). And then we employed wound healing method to examine the migration of CCAT1-depleted ovarian cancer cells SKOV3 and CaOV3 in the presence of TGFβ1(10 ng/ml). The results showed that scramble (sh-NC) cells exhibited no migration difference compared to control, whereas CCAT1 knockdown (sh-CCAT1) cell significantly compromised migration (reduced by ~ 40–50%) of ovarian cancer cells induced by TGFβ1 in relative to sh-NC group (Fig. 1c, d). In addition, we sought to determine whether TGFβ1-induced invasion of ovarian cells were regulated by CCAT1 loss. Transwell matrix penetration assay demonstrated that knockdown of CCAT1 reduced the number of invasive ovarian cancer cells (by ~ 50%) in the presence of TGFβ1 compared to sh-NC group (Fig. 1e). To further investigate the mechanism underlying TGFβ1-induced invasion and migration of ovarian cancer cells regulated by CCAT1, we examined the expression of EMT-associated genes in control and sh-CCAT1 ovarian cells. RT-qPCR and western blot analyses showed that CCAT1 knockdown markedly attenuated TGFβ1-induced expression of vimentin, N-cadherin and MMP9, on the other hand, CCAT1 knockdown enhanced TGFβ1-induced expression of E-cadherin and Claudin (Fig. 1f, g). Taken together, these results revealed that CCAT1 loss led to remarkably attenuated EMT of ovarian cancer cells treated with TGFβ1.
LncRNA CCAT1 depletion attenuates TGFβ1-induced EMT of ovarian cancer cells. a The level of CCAT1 in ovarian cancer cells (SKOV3 and CaOV3) was detected by RT-qPCR at 48 h after treated with TGFβ1 (10 ng/ml). b RT-qPCR analysis was performed to confirm target gene CCAT1 silencing after shRNA treatment. RNA level of CCAT1 in sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) were detected in ovarian cancer cells (SKOV3 and CaOV3). c, d Representative images of wound healing assay (c) and quantification (d) carried out in control (wildtype), sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) ovarian cancer cells (SKOV3 and CaOV3) treated with 10 ng/ml TGFβ1. e Cell invasion ability of control (wildtype), sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) ovarian cancer cells (SKOV3 and CaOV3) was measured with transwell invasion assay under 10 ng/ml TGFβ1. f the mRNA level of EMT-associated markers (claudin, E-cadherin, N-cadherin, vimentin and MMP9) in control (wildtype), sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) ovarian cancer cells (SKOV3 and CaOV3) was measured by RT-qPCR analysis after cells treated with 10 ng/ml TGFβ1. g Western blot analysis showed the protein level of EMT-associated markers (claudin, E-cadherin, N-cadherin, vimentin and MMP9) after cells were transfected with sh-CCAT1 (CCAT1 knockdown) in ovarian cancer cells (SKOV3 and CaOV3) compared with the control (wildtype), sh-NC (scramble) groups under with 10 ng/ml TGFβ1. All data were represented as mean ± SD from three biological replicates (*P < 0.05; **P < 0.01)
LncRNA CCAT1 depletion decreases expression level of TGFβR1
In order to clarify the mechanism by which CCAT1 loss compromised TGFβ1-induced EMT, we hypothesized that TGFβ1 cognate receptor, TGFβR1, might be regulated by CCAT1. Interestingly, we observed that TGFβR1 mRNA level was diminished by about 50–70% in CCAT1-null cells compared to shNC cells (Fig. 2a). In agreement with this, protein level of TGFβR1 was also downregulated by CCAT1 depletion in both SKOV3 and CaOV3 cells (Fig. 2b). In sum, these data indicated that CCAT1 played its roles in TGFβ1-induced EMT of ovarian tumor through enhancing TGFβR1 expression.
lncRNA CCAT1 depletion decreases expression level of TGFβR1. a RT-qPCR analysis showed the mRNA level of TGFβR1 in sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) ovarian cancer cells (SKOV3 and CaOV3). b The protein level of TGFβR1 in sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) ovarian cancer cells (SKOV3 and CaOV3) was detected by western blot. All data were represented as mean ± SD from three biological replicates (*P < 0.05)
LncRNA CCAT1 directly targets miR-490-3p
It has been reported that miR-490-3p has been implicated in ovarian tumor invasion and metastasis [31]. We reasoned that CCAT1 might exert its role by regulating miR-490-3p in ovarian cancer cells. Bioinformatic analysis revealed that CCAT1 could directly target miR-490-3p by matching with sequence at 3′-terminus (Fig. 3a). Furthermore, luciferase reporter assay was used to confirm that CCAT1 directly interacted with miR-490-3p. We found that miR-490-3p overexpression only decreased wildtype CCAT1-fused luciferase activity, not CCAT1 mutant (Fig. 3b). Notably, miR-490-3p level was substantially upregulated by CCAT1 knockdown in SKOV3 and CaOV3 cells (Fig. 3c). Moreover, miR-490-3p mimics suppressed CCAT1 expression, and instead miR-490-3p inhibitor enhanced CCAT1 expression of SKOV3 and CaOV3 cells (Fig. 3d). RT-qPCR and western blot analyses showed that miR-490-3p overexpression augmented CCAT1 depletion-induced TGFβR1 downregulation, while miR-490-3p inhibitor greatly restored the expression level of TGFβR1 (Fig. 3e, f). Together, these results suggested that lncRNA CCAT1 loss downregulated TGFβR1 expression via directly targeting miR-490-3p.
LncRNA CCAT1 directly targets miR-490-3p. a Diagram of bioinformatic prediction of binding site of miR-490-3p by CCAT1. b Cells were co-transfected with miR-490-3p mimics and wildtype CCAT1 or mutant CCAT1luciferase reporter plasmid. The cell lysates were harvested for luciferase assay. c RT-qPCR analysis showed the level of miR-490-3p in sh-NC (scramble) and sh-CCAT1 (CCAT1 knockdown) ovarian cancer cells (SKOV3 and CaOV3). d The level of CCAT1 of ovarian cancer cells which transfected with miR-490-3p mimic and miR-490-3p inhibitor was detected by RT-qPCR. e RT-qPCR analysis showed the mRNA level of TGFβR1 in ovarian cancer cells (SKOV3 and CaOV3) transfected with sh-NC (scramble), sh-CCAT1 (CCAT1 knockdown), sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p mimics, sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p inhibitor. f The protein level of TGFβR1 in ovarian cancer cells (SKOV3 and CaOV3) transfected with sh-NC (scramble), sh-CCAT1 (CCAT1 knockdown), sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p mimics, sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p inhibitor was measured by western lot assay. All data were represented as mean ± SD from three biological replicates (*P < 0.05; **P < 0.01)
miR-490-3p is essential for TGFβ1-induced EMT affected by CCAT1 knockdown via downregulation of TGFβR1
As CCAT1 was shown to decrease miR-490-3p level, then upregulating TGFβR1 expression in SKOV3 and CaOV3 cells; therefore, we sought to assess whether miR-490-3p was essential for CCAT1-mediated tumor phenotypes of ovarian cancer cells. Wound healing assay showed that CCAT1 knockdown alone impaired (decreased by 52%) the ability of TGFβ1 to promote cells migration. Transfection of both miR-490-3p mimics and CCAT1 shRNA markedly attenuated (dropped by ~ 88%) migration of ovarian cancer cells relative to shCCAT1 alone, while co-transfection of miR-490-3p inhibitor and CCAT1 shRNA into cells exhibited more robust migration (increased by ~ 72%) than shCCAT1-expressing cells (Fig. 4a). Besides, we found that miR-490-3p mimics potentiated shCCAT1-inhibited invasiveness (the invasion cell number decreased by 60%) and instead miR-490-3p inhibitor attenuated (the invasived cell number increased by 61%) shCCAT1-inhibited invasiveness of SKOV3 and CaOV3 cells (Fig. 4b). As for EMT-associated markers, RT-qPCR and westernblot revealed that upregulation of E-cadherin and Claudin by CCAT1 loss was enhanced by miR-490-3p mimics and attenuated by miR-490-3p inhibitor in SKOV3 and CaOV3 cells, inverse in the expression of vimentin, N-cadherin and MMP9 (Fig. 4c, d). Collectively, these findings indicated that miR-490-3p was essential for TGFβ1-induced EMT of ovarian cancer cells regulated by CCAT1 depletion.
miR-490-3p is essential for TGFβ1-induced EMT affected by CCAT1 knockdown via downregulation of TGFβR1. a Representative images of wound healing assay and quantification carried out in ovarian cancer cells (SKOV3 and CaOV3) transfected with sh-NC (scramble), sh-CCAT1 (CCAT1 knockdown), sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p mimics, sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p inhibitor. All cells were treated with 10 ng/ml TGFβ1. b Cell invasion assay and quantification showed invasiveness of ovarian cancer cells (SKOV3 and CaOV3) transfected with sh-NC (scramble), sh-CCAT1 (CCAT1 knockdown), sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p mimics, sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p inhibitor. All cells were treated with 10 ng/ml TGFβ1. c The mRNA level of TGFβR1 and EMT-associated markers (claudin, E-cadherin, N-cadherin, vimentin and MMP9) in ovarian cancer cells (SKOV3 and CaOV3) transfected with sh-NC (scramble), sh-CCAT1 (CCAT1 knockdown), sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p mimics, sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p inhibitor was detected by RT-qPCR. All cells were treated with 10 ng/ml TGFβ1. d Westernblot analysis showed the protein level of TGFβR1 and EMT-associated markers (claudin, E-cadherin, N-cadherin, vimentin and MMP9) in ovarian cancer cells (SKOV3 and CaOV3) transfected with sh-NC (scramble), sh-CCAT1 (CCAT1 knockdown), sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p mimics, sh-CCAT1 (CCAT1 knockdown) plus miR-490-3p inhibitor. All cells were treated with 10 ng/ml TGFβ1. All data were represented as mean ± SD from three biological replicates (*P < 0.05; **P < 0.01)
miR-490-3p inhibits TGFβ1-induced EMT through directly targeting TGFβR1
To further explore how miR-490-3p affected CCAT1 loss-induced TGFβR1 expression, we inferred that miR-490-3p probably targeted TGFβR1 and regulated its expression. The bioinformatic analyses revealed that miR-490-3p could target 3′-UTR of TGFβR1 mRNA (Fig. 5a). Luciferase reporter assay demonstrated that miR-490-3p mimics effectively inhibited the activity in wildtype 3′-UTR of TGFβR1 cells, not the mutant, suggesting miR-490-3p played its role via directly binding 3′-UTR of TGFβR1 (Fig. 5b).
miR-490-3p inhibited TGFβ1-induced EMT through directly targeting TGFβR1. a Diagram of bioinformatic prediction of binding site of TGFβR1 by miR-490-3p. b Cells were cotransfected with scrambled RNA or miR-490-3p together with TGFβR1-3′-UTR or TGFβR1-mut-3′-UTR luciferase reporter in the presence of firefly luciferase reporter plasmid. Renilla luciferase activity and firefly luciferase activity were measured by dual-luciferase reporter assay. Renilla luciferase activity was normalized to firefly luciferase activity. c The mRNA level of TGFβR1 in ovarian cancer cells (SKOV3 and CaOV3) transfected with negative control, miR-490-3p mimics, miR-490-3p mimics plus TGFβR1 was detected by RT-qPCR analysis. d Westernblot analysis showed the protein level of TGFβR1 in ovarian cancer cells (SKOV3 and CaOV3) transfected with negative control, miR-490-3p mimics, miR-490-3p mimics plus TGFβR1. β-actin as loading control. e Representative images of wound healing assay and quantification carried out in negative control, miR-490-3p mimics or miR-490-3p mimics- plus TGFβR1-overexpressing ovarian cancer cells (SKOV3 and CaOV3) treated with 10 ng/ml TGFβ1. f Cell invasion assay and quantification showed invasiveness of negative control, miR-490-3p mimics or miR-490-3p mimics plus TGFβR1 overexpressing ovarian cancer cells (SKOV3 and CaOV3) treated with 10 ng/ml TGFβ1. g Westernblot analysis showed the protein level of EMT-associated markers (claudin, E-cadherin, N-cadherin, vimentin and MMP9) in negative control, miR-490-3p mimics or miR-490-3p mimics- plus TGFβR1-overexpressing ovarian cancer cells (SKOV3 and CaOV3). All cells were treated with 10 ng/ml TGFβ1. All data were represented as mean ± SD from three biological replicates (*P < 0.05; **P < 0.01)
To determine the biological function of miR-490-3p-induced TGFβR1 downregulation, we overexpressed miR-490-3p mimics alone or in combination with TGFβR1. The RT-qPCR and westernblot analyses showed TGFβR1 was reduced by miR-490-3p mimics overexpression; however, TGFβR1 level increased when the cells overexpressing miR-490-3p and TGFβR1 (Fig. 5c, d). Next, we observed that miR-490-3p overexpression greatly attenuated migration of ovarian cancer cells SKOV3 and CaOV3, whereas exogenous expression of miR-490-3p and TGFβR1 rescued TGFβ1-induced migration change of the cells (Fig. 5e, f). Similarly, miR-490-3p mimics caused remarkably decreased invasion of ovarian cancer cells compared to negative control, and transfection of miR-490-3p plus TGFβR1 could enhance the invasiveness of ovarian cancer cells comparable to control (Fig. 5f). Finally, we detected EMT-associated markers after overexpression of miR-490-3p and TGFβR1. Our results showed miR-490-3p inhibited TGFβ1-induced expression of vimentin, N-cadherin and MMP9, instead, upregulated TGFβ1-induced expression of E-cadherin and Claudin. More importantly, overexpression of TGFβR1 reverted miR-490-3p-mediated regulation of EMT-related genes in ovarian cancer cells (Fig. 5g).
LncRNA CCAT1 negatively correlates with miR-490-3p level in ovarian tumors
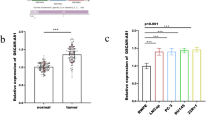
To determine the clinical association of CCAT1 and miR-490-3p expression with progression of ovarian cancer, we examined CCAT1 and miR-490-3p expression level of ovarian tumors (n = 25) and adjacent normal tissues (n = 25) by RT-qPCR approach. CCAT1 level was higher (~ 2.6 folds) in tumors than in normal tissues; however, miR-490-3p level was lower (~ 65%) in tumors compared to normal tissues (Fig. 6a, b). In addition, the bioinformatic analysis showed negative association between CCAT1 and miR-490-3p expression in ovarian tumors (r2 = 0.8579, p < 0.01) (Fig. 6c). To summarize, our data indicated that CCAT1, clinically, might promote ovarian cancer via inhibiting expression of miR-490-3p.
LncRNA CCAT1 negatively correlates with miR-490-3p level in ovarian tumors. a The level of CCAT1 in ovarian tumors or adjacent tissues (normal) was measured by RT-qPCR, n = 25. b The level of miR-490-3p in ovarian tumors or adjacent tissues (normal) was detected by RT-qPCR analysis, n = 25. c Pearson’s correlation analysis was used to determine the correlation between the expression levels of CCAT1 and miR-490-3p in human ovarian tumors; Spearman’s correlation, r2 = 0.8597(n = 25). All data were represented as mean ± SD (*P < 0.05)
Discussion
Ovarian cancer results in the death of about 140,000 women, and limited improvement of survival rate has been achieved in ovarian cancer [1]. Most patients with ovarian cancer died from advanced stage (metastatic) of the cancer, other than early stage [4]. Therefore, it is key to illuminate the mechanism underlying metastasis of ovarian cancer. In this study, our results demonstrated that lncRNA CCAT1 enhanced TGFβ1-induced metastatic process of ovarian cancer cells via miR-490-3p/TGFβR1 axis, which was crucial for developing targeted drugs for treating ovarian cancer patients with advanced stage.
TGFβ1 signaling is important in a number of cellular processes, physiologically and pathologically [32]. And it is believed that TGFβ1 switches its suppressive role in normal cells into tumor-stimulatory role in cancer cells. Such as, TGFβ1 could induce EMT and metastasis of human ovarian cancer cells [12]. More interestingly, TGFβ1 could modulate EMT by impacting expression of lncRNAs and miRNAs in gastric cancer and bladder cancer. For example, TGFβ1-induced LncRNA UCA1 upregulation promotes gastric cancer invasion and migration [33]. In addition, TGFβ1 secreted by cancer-associated fibroblasts induces EMT of bladder cancer cells through lncRNA-ZEB2NAT [34]. In this study, we first proved that TGFβ1 upregulated expression of lncRNACCAT1 in ovarian cancer cells and knockdown of CCAT1 inhibited TGFβ1-induced EMT. Moreover, consistent with previous studies LncRNA CCAT1 promotes EMT of intrahepatic cholangiocarcinoma [35]. In addition, it was reported that LncRNA CCAT1 promoted EMT of epithelial ovarian cancer cells via miR-152/miR-130-Zeb1 axis [21]. All these revealed that TGFβ1 induced EMT of ovarian cancer partly dependent on lncRNACCAT1.
Emerging evidence has revealed that lncRNAs exert its effects as competing endogenous RNA (ceRNA) [30]. In the case, lncRNAs commonly interact with miRNAs and mutually regulate each other’s expression. LncRNAs function as ceRNAs to target and degrade miRNAs; however, miRNAs suppress lncRNA through an Argonaute 2-mediated pathway [36, 37]. In the previous report, it was found that CCAT1 is a driver of malignancy, which acts in part through ‘sponging’ miRNA-218-5p in gallbladder cancer [18]. It was also found that CCAT1 could target and sponge miR-152 in ovarian cancer cells [21]. In this study, we found that CCAT1 function as ceRNA to directly bind and decline miR-490-3p via complementary sequence. Consistent with the reports that the long noncoding RNA colon cancer-associated transcript-1/miR-490 axis regulates gastric cancer cell migration by targeting hnRNPA1 [30]. MiR-490-3p has been reported to act as oncosuppressive microRNA to inhibit breast cancer tumorigenesis and progression by targeting RhoA directly [38]. Importantly, miR-490-3p may target CDK1 and inhibit ovarian epithelial carcinoma tumorigenesis and progression [31]. Consistent with these results, functionally, we observed that miR-490-3p overexpression led to attenuated migration and invasion, and regulated EMT-associated genes (vimentin, N-cadherin, E-cadherin and Claudin). These data imply that knockdown CCAT1 inhibited TGFβ1-induced EMT in ovarain cancer cells through sponging miR-490-3p.
Xuehu Xu et al. observed that miR-490-3p targeted TGFβR1 to inhibit colorectal cancer metastasis [29]. Consistently, our results revealed that miR-490-3p suppressed TGFβR1 expression and TGFβR1 overexpression could rescue miR-490-3p-inhibited EMT. J Xiang et al. reported that TGFβR1 promoted EMT of gastric cancer treated with TGFβ, which was attenuated by Grhl2 [39]. Besides, 14-3-3/TGFβR1 axis also promoted tumor metastasis in lung squamous carcinoma [40]. Hence, these conclusions further support our notion described above.
Conclusion
Here, our results demonstrated that lncRNA CCAT1 enhanced TGFβ1-induced metastatic process of ovarian cancer cells via miR-490-3p/TGFβR1 axis in ovarian cancer cells. This new molecular axis was confirmed to be important for TGFβ1-induced EMT of ovarian cancer; however, other possible mechanisms responsible for CCAT1-mediated metastasis of ovarian cancer cells remains to be investigated for the future. Our findings shed lights on how CCAT1 regulates TGFβ1-promoted cancer metastasis and facilitate development of effective therapies for treating ovarian cancer.
Abbreviations
- LncRNA:
-
long noncoding RNA
- EMT:
-
epithelial–mesenchymal transition
- CCAT1:
-
colon cancer-associated transcript 1
- TGFβ1:
-
transforming growth factor-β1
- TGFβR1:
-
transforming growth factor-β receptor 1
- MAPK:
-
mitogen-activated protein kinase
- ZEB1/2:
-
zinc finger E-box-binding homeobox1/2
- 3′-UTR:
-
3′-untranslated region
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- ATCC:
-
American Tissue Culture Collection
- siRNA:
-
short interference RNA
- shRNA:
-
short hairpin RNA
- MMP9:
-
matrix metalloproteinase9
- ceRNA:
-
competing endogenous RNA
- RT-qPCR:
-
reverse transcription quantitative real time polymerase chain reaction
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Davidson B, Trope CG. Ovarian cancer: diagnostic, biological and prognostic aspects. Women’s Health. 2014;10(5):519–33.
Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin Cancer Res. 2014;20(20):5150–6.
Shapira I, Oswald M, Lovecchio J, Khalili H, Menzin A, Whyte J, Dos Santos L, Liang S, Bhuiya T, Keogh M, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110(4):976–83.
Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25(1):76–84.
Moustakas A, Heldin CH. Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–20.
Moustakas A, Heldin CH. Induction of epithelial–mesenchymal transition by transforming growth factor beta. Semin Cancer Biol. 2012;22(5–6):446–54.
Hoot KE, Lighthall J, Han G, Lu SL, Li A, Ju W, Kulesz-Martin M, Bottinger E, Wang XJ. Keratinocyte-specific Smad2 ablation results in increased epithelial–mesenchymal transition during skin cancer formation and progression. J Clin Investig. 2008;118(8):2722–32.
Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178(3):437–51.
Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial–mesenchymal transition and cell invasion. J Cell Sci. 2012;125(Pt 5):1259–73.
Li W, Zhang X, Wang J, Li M, Cao C, Tan J, Ma D, Gao Q. TGFbeta1 in fibroblasts-derived exosomes promotes epithelial–mesenchymal transition of ovarian cancer cells. Oncotarget. 2017;8(56):96035–47.
Teng Y, Zhao L, Zhang Y, Chen W, Li X. Id-1, a protein repressed by miR-29b, facilitates the TGFbeta1-induced epithelial–mesenchymal transition in human ovarian cancer cells. Cell Physiol Biochem. 2014;33(3):717–30.
Wu P, Zuo X, Deng H, Liu X, Liu L, Ji A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Res Bull. 2013;97:69–80.
Wu Z, Liu X, Liu L, Deng H, Zhang J, Xu Q, Cen B, Ji A. Regulation of lncRNA expression. Cell Mol Biol Lett. 2014;19(4):561–75.
Wu Z, Wu P, Zuo X, Yu N, Qin Y, Xu Q, He S, Cen B, Liao W, Ji A. LncRNA-N1LR enhances neuroprotection against ischemic stroke probably by inhibiting p53 phosphorylation. Mol Neurobiol. 2016;54:7670–85.
Alaiyan B, Ilyayev N, Stojadinovic A, Izadjoo M, Roistacher M, Pavlov V, Tzivin V, Halle D, Pan H, Trink B, et al. Differential expression of colon cancer associated transcript1 (CCAT1) along the colonic adenoma-carcinoma sequence. BMC cancer. 2013;13:196.
Dou C, Sun L, Jin X, Han M, Zhang B, Li T. Long non-coding RNA colon cancer-associated transcript 1 functions as a competing endogenous RNA to regulate cyclin-dependent kinase 1 expression by sponging miR-490-3p in hepatocellular carcinoma progression. Tumour Biol. 2017;39(4):1010428317697572.
Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W, Quan ZW. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583.
Yang F, Xue X, Bi J, Zheng L, Zhi K, Gu Y, Fang G. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139(3):437–45.
Ye Z, Zhou M, Tian B, Wu B, Li J. Expression of lncRNA-CCAT1, E-cadherin and N-cadherin in colorectal cancer and its clinical significance. Int J Clin Exp Med. 2015;8(3):3707–15.
Cao Y, Shi H, Ren F, Jia Y, Zhang R. Long non-coding RNA CCAT1 promotes metastasis and poor prognosis in epithelial ovarian cancer. Exp Cell Res. 2017;359(1):185–94.
Calame K. MicroRNA-155 function in B Cells. Immunity. 2007;27(6):825–7.
Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38(1):17–26.
Zaravinos A. The regulatory role of MicroRNAs in EMT and cancer. J Oncol. 2015;2015:865816.
Zhao K, Wang L, Li T, Zhu M, Zhang C, Chen L, Zhao P, Zhou H, Yu S, Yang X. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int J Oncol. 2017;50(6):1989–99.
Wang X, Liang Z, Xu X, Li J, Zhu Y, Meng S, Li S, Wang S, Xie B, Ji A, et al. miR-148a-3p represses proliferation and EMT by establishing regulatory circuits between ERBB3/AKT2/c-myc and DNMT1 in bladder cancer. Cell Death Dis. 2016;7(12):e2503.
Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M, Shao Z, Zhang F, Luo Y, Shen Z, et al. Epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/beta-catenin signaling pathway. Cancer Lett. 2016;376(1):178–87.
Zhang LY, Liu M, Li X, Tang H. miR-490-3p modulates cell growth and epithelial to mesenchymal transition of hepatocellular carcinoma cells by targeting endoplasmic reticulum-Golgi intermediate compartment protein 3 (ERGIC3). J Biol Chem. 2013;288(6):4035–47.
Xu X, Chen R, Li Z, Huang N, Wu X, Li S, Li Y, Wu S. MicroRNA-490-3p inhibits colorectal cancer metastasis by targeting TGFbetaR1. BMC Cancer. 1023;2015:15.
Zhou B, Wang Y, Jiang J, Jiang H, Song J, Han T, Shi J, Qiao H. The long noncoding RNA colon cancer-associated transcript-1/miR-490 axis regulates gastric cancer cell migration by targeting hnRNPA1. IUBMB Life. 2016;68(3):201–10.
Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer Lett. 2015;362(1):122–30.
Alsina-Sanchis E, Figueras A, Lahiguera A, Gil-Martin M, Pardo B, Piulats JM, Marti L, Ponce J, Matias-Guiu X, Vidal A, et al. TGFbeta controls ovarian cancer cell proliferation. Int J Mol Sci. 2017;18(8):1658.
Zuo ZK, Gong Y, Chen XH, Ye F, Yin ZM, Gong QN, Huang JS. TGFbeta1-induced LncRNA UCA1 upregulation promotes gastric cancer invasion and migration. DNA Cell Biol. 2017;36(2):159–67.
Zhuang J, Lu Q, Shen B, Huang X, Shen L, Zheng X, Huang R, Yan J, Guo H. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial–mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Scientific Rep. 2015;5:11924.
Zhang S, Xiao J, Chai Y, Du YY, Liu Z, Huang K, Zhou X, Zhou W. LncRNA-CCAT1 promotes migration, invasion, and EMT in intrahepatic cholangiocarcinoma through suppressing miR-152. Dig Dis Sci. 2017;62(11):3050–8.
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–69.
Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al. In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell. 2011;147(2):382–95.
Zhao L, Zheng XY. MicroRNA-490 inhibits tumorigenesis and progression in breast cancer. Onco Targets Ther. 2016;9:4505–16.
Xiang J, Fu X, Ran W, Wang Z. Grhl2 reduces invasion and migration through inhibition of TGFbeta-induced EMT in gastric cancer. Oncogenesis. 2017;6(1):e284.
Zhao Y, Qiao W, Wang X, Yin H, Cui J, Cui Y, Chen X, Hu J, Lu H, Meng Q, et al. 14-3-3zeta/TGFbetaR1 promotes tumor metastasis in lung squamous cell carcinoma. Oncotarget. 2016;7(50):82972–84.
Authors’ contributions
Guarantor of integrity of the entire study: CYL, study concepts: MY, study design: CYL, definition of intellectual content: MY, literature research: MY, clinical studies: MY, experimental studies: MY, data acquisition: LN, data analysis: LN, statistical analysis: MY, manuscript preparation: MY, manuscript editing: CYL, manuscript review: CYL. All authors read and approved the final manuscript.
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
One-way ANOVA was used for comparison among multiple groups and multiple comparisons were further performed using post hoc Turkey test.
Consent for publication
All authors agree to submit this manuscript and declare no competing of interest related to this work.
Ethics approval and consent to participate
This experiment was approved by ethic committee of the 2nd Affiliated Hospital of Harbin Medical University, and the tissues were acquired with the consent of patients.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mu, Y., Li, N. & Cui, YL. The lncRNA CCAT1 upregulates TGFβR1 via sponging miR-490-3p to promote TGFβ1-induced EMT of ovarian cancer cells. Cancer Cell Int 18, 145 (2018). https://doi.org/10.1186/s12935-018-0604-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-018-0604-1