Abstract
Background
The effect of change in blood glucose levels on the risk of cardiovascular disease among individuals without diabetes is currently unclear. We aimed to examine the association of change in fasting serum glucose with incident cardiovascular disease and all-cause mortality among representative large population.
Methods
We analyzed the data from retrospective cohort of Korean National Health Insurance Service. In total, 260,487 Korean adults aged over 40 years, without diabetes mellitus and cardiovascular disease at baseline measured change in fasting serum glucose according to the criteria of impaired and diabetic fasting glucose status: normal fasting glucose (NFG, fasting glucose: < 100 mg/dL), impaired fasting glucose (IFG, fasting glucose: 100.0–125.9 mg/dL), and diabetic fasting glucose (DFG, fasting glucose: ≥ 126.0 mg/dL). Compared to the persistently unchanged group (i.e. NFG to NFG or IFG to IFG), Cox proportional hazards regression analyses were performed in the changed group to obtain the hazards ratio (HR) with 95% confidence interval (CI) for the subsequent median 8-year myocardial infarction, stroke, and all-cause mortality.
Results
Compared to individuals with persistent NFG (i.e., NFG to NFG), individuals who shifted from NFG to DFG had an increased risk of stroke (HR [95% CI]: 1.19 [1.02–1.38]) and individuals who shifted from NFG to IFG or DFG had increased risks of all-cause mortality (HR [95% CI]: 1.08 [1.02–1.14] for NFG to IFG and 1.56 [1.39–1.75] for NFG to DFG). Compared to individuals with persistent IFG, individuals who shifted from IFG to DFG had an increased risk of MI and all-cause mortality (HR [95% CI]: 1.65 [1.20–2.27] and 1.16 [1.02–1.33], respectively).
Conclusions
Increasing fasting glucose in non-diabetic population is associated with risks of the MI, stroke, and all-cause mortality, which is more rapid, more severe.
Similar content being viewed by others
Background
Diabetes is related to both microvascular and macrovascular complications as well as mortality [1, 2]. In particular, macrovascular complications such as myocardial infarction (MI) and stroke account for 80% of all deaths in patients with type 2 diabetes mellitus [3]. Therefore, preventing macrovascular complications by controlling hyperglycemia is imperative in reducing the risk of cardiovascular disease and mortality [4,5,6].
Recent evidence suggests that a prediabetic status may also elevate the risk of cardiovascular disease and mortality. A prediabetic status referred to people who have impaired fasting glucose (IFG, fasting glucose of 100–125 mg/dL), impaired glucose tolerance (IGT, 2-h post glucose load of 140–199 mg/dL), or both, according to American Diabetes Association [5]. Some studies have shown that IGT is superior to IFG as a risk factor for cardiovascular disease [7,8,9,10]. However, the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) reported IFG as well as IGT to be an independent predictor of cardiovascular disease and all-cause mortality [11]. Moreover, meta-analyses also showed that not only IGT but IFG is related to risk of cardiovascular disease [12, 13].
Blood glucose levels in most prior studies, however, were assessed only one time, possibly resulting in misclassification of IFG or IGT. The effect of change in blood glucose levels on the risk of cardiovascular disease among people without diabetes are currently unclear. We, therefore, aimed to examine the association of change in fasting serum glucose with incident cardiovascular disease and all-cause mortality within a large population without diabetes using the Korean National Health Insurance Service-Nation Health Screening Cohort (NHIS-HEALS).
Methods
Study population
The study population was derived from the NHIS-HEALS database from January 1, 2002 to December 31, 2013, which is provided by the NHIS (NHIS-2017-2-460). In 2000, NHIS was launched by integrating diverse medical insurance organizations in Korea. The NHIS has provided mandatory health insurance for all Koreans since 1989; thus, the enrollment rate is nearly 98% [14]. Attrition over follow-up in this database is known to be rare because the NHIS acts as a universal health insurance [15]. Enrollees over 40 years of age are required to take bi-annual health screening visits, including gathering of clinical and sociodemographic data. Among the entire data set from the national health examination, 10% (about 500,000 participants) of the data are collected by simple random sampling with deidentification [15]. NHIS-HEALS contains retrospective data, including electronic medical records, information on clinical visits, diagnosis based on International Classification of Disease (ICD) codes, anthropometry, and laboratory examination. Sociodemographic data on age, sex, income status, residential information, and disability status are also included. The NHIS plays a role in paying premiums to medical institutions. For these reasons, data are known to be accurate [15].
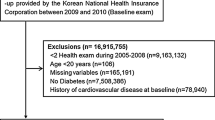
A total of 334,377 participants who took at least two visits of health screening between the first (from 2002 to 2003) and second (from 2004 to 2005) health examinations, with fasting serum glucose values, were selected. Among these individuals, we excluded 42,097 participants who were diagnosed with type 2 diabetes before the onset of follow-up (index date: January 1, 2006) according to the ICD-10 codes (10th revision) by the World Health Organization for type 2 diabetes (E11, E12, E14) or those with baseline fasting glucose levels ≥ 126.0 mg/dL at their first health examination. We further excluded 780 participants who passed away and 30,753 participants who were diagnosed MI or stroke before the index date. Then, we removed 122 participants with fasting glucose < 50.0 mg/dL and 138 participants without sex values. Finally, the study population consisted of 260,487 participants (149,913 men, 110,574 women).
Data collection
The participants underwent blood examination including fasting glucose levels during each health visit. We measured changes in fasting serum glucose levels from the first health examination to the second health examination in this study (Fig. 1). Fasting glucose levels of the first health examination were divided into two groups: normal fasting glucose (NFG, fasting glucose: < 100 mg/dL) and impaired fasting glucose (IFG, fasting glucose: 100.0–125.9 mg/dL). Fasting glucose levels of the second health examination were divided into 3 groups: NFG, IFG, and diabetic fasting glucose (DFG, fasting glucose: ≥ 126.0 mg/dL). The changes in fasting glucose were defined as the shift from each fasting glucose level of the first health examination (baseline) to each fasting glucose of the second health examination after 2 years.
Timeline of the study design. Subjects without diabetes and cardiovascular disease performed 2-year fasting serum glucose examination. The first fasting glucose status was categorized into two groups, normal fasting glucose [(NFG), fasting serum glucose: < 100.0 mg/dL] and impaired fasting glucose [(IFG), fasting serum glucose: 100.0–125.9 mg/dL] (individuals with more than 126 mg/dL were excluded). The second fasting glucose status was categorized into three groups, NFG, IFG, and diabetic fasting glucose [(DFG), fasting serum glucose: ≥ 126.0 mg/dL]. Accordingly, six categories based on the change in fasting glucose level were followed up during 8 years for determining the risk of myocardial infarction (MI), stroke, and all-cause mortality
The main outcomes of this study were hospitalizations due to MI or stroke, and all-cause mortality that occurred from January 1, 2006 and December 31, 2013. ICD-10 codes were used to identify and classify the outcomes: MI (I21–I24), stroke (I60–69). Hospitalization due to cardiovascular events was defined as participants who were hospitalized for 2 days or more due to the relevant disease, in order to exclude admissions for other diseases.
Covariates were based on the data from near before index year included age, sex, socioeconomic status (low and high), body mass index (BMI, kg/m2), smoking status (never and ever), alcohol consumption (none, < 3, and ≥ 3 times per week), physical activity (no, < 3, and ≥ 3 times per week), systolic/diastolic blood pressure (mmHg), total cholesterol (mg/dL), Charlson Comorbidity Index (CCI, 0, 1–2, and ≥ 3). Participants were classified as underweight, normal weight, overweight, and obese based on the Asian criteria [16]. CCI is the most commonly used comorbidity index for predicting mortality [17, 18].
Statistical analysis
Cox proportional hazards regression analyses were performed to obtain the hazards ratio (HR) with 95% confidence interval (CI) of MI, stroke, and all-cause mortality for each NFG and IFG group at the first health examination, after adjusting for age, sex, socioeconomic status, BMI, smoking status, alcohol consumption, physical activity, CCI, blood pressure, total cholesterol, and baseline fasting glucose level. Among covariates, age, BMI, blood pressure, total cholesterol and fasting glucose were dealt with continuous variables. Those with persistently unchanged fasting glucose (NFG at the first health examination to NFG at the second health examination and IFG at the first health examination to IFG at the second health examination) were considered the reference group. Stratified analyses were conducted including age, sex, and BMI in order to identify potential subgroups that show a significant association between change in fasting glucose and MI, stroke, and all-cause mortality. After excluding participants who were diagnosed with myocardial infarction or stroke, or who died between January 1, 2006 and December 31, 2006, and between January 1, 2006 and December 31, 2007, sensitivity analyses were performed to enhance the reliability of the association between change in fasting glucose levels and MI, stroke, and mortality. All data mining and statistical analyses in this study were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) and STATA 13.0 (StataCorp LP, College Station, TX, USA). Statistical significance of this study was defined as a two-sided p value less than 0.05.
Results
260,487 individuals without diabetes were followed up to an average of 8 years (standard deviation, SD 0.9), resulting in 2,042,960 person-years. During the follow-up, the number of incident MI, incident stroke, and all-cause mortalities were 1318 (0.5%), 8144 (3.13%), 10,065 (3.86%), respectively. The baseline characteristics of study participants are presented in Table 1. Those who belonged to the NFG group at the baseline were more likely to be younger women, with a lower BMI, never smokers, with a lower alcohol consumption, lower blood pressure, and lower total cholesterol than those who were in the IFG group at baseline.
Figure 2 shows adjusted cumulative hazard curves for 8-year MI, stroke, and all-cause mortality by changes in fasting glucose, after adjusting for age, socioeconomic status, smoking habit, alcohol consumption, physical activity, BMI, CCI, blood pressure, and fasting glucose at baseline. Compared to individuals with persistent NFG (i.e., NFG to NFG), individuals who shifted from NFG to DFG had an increased risk of stroke (HR [95% CI]: 1.19 [1.02–1.38]) and individuals who shifted from NFG to IFG or DFG had increased risks of all-cause mortality (HR [95% CI]: 1.08 [1.02–1.14] for NFG to IFG and 1.56 [1.39–1.75] for NFG to DFG). Compared to individuals with persistent IFG, individuals who shifted from IFG to DFG had an increased risk of MI and all-cause mortality (HR [95% CI]: 1.65 [1.20–2.27] and 1.16 [1.02–1.33], respectively).
Adjusted cumulative hazard curves for 8-year MI, stroke, and all-cause mortality by change in fasting glucose. Hazard ration analyzed by Cox proportional hazards regression analysis adjusted for age, sex, socioeconomic status, physical activity, smoking status, alcohol consumption, body mass index, blood pressure, total cholesterol, Charlson comorbidity index, and baseline fasting glucose level. MI myocardial infarction, NFG normal fasting glucose, IFG impaired fasting glucose, DFG diabetic fasting glucose. a Cumulative hazard of MI among individuals with NFG at the 1st examination. b Cumulative hazard of stroke among individuals with NFG at the 1st examination. c Cumulative hazard of all-cause mortality among individuals with NFG at the 1st examination. d Cumulative hazard of MI among individuals with IFG at the 1st examination. e Cumulative hazard of stroke among individuals with IFG at the 1st examination. f Cumulative hazard of all-cause mortality among individuals with IFG at the 1st examination
Subgroup analyses using the multivariable model of MI, stroke, and all-cause mortality risks by changes in fasting glucose from NFG and IFG are shown in Tables 2, 3, 4. Individuals who were 65 years or older had an increased risk for MI. With regard to all-cause mortality, results of all subgroups stratified by age, sex, and BMI were similar to the main results. The magnitude of HRs of all-cause mortality was higher in patients who were more than 50 years old than in those less than 50 years, in men than in women, and those with BMI ≥ 25 kg/m2 (obese based on Asian criteria) than with BMI < 25 kg/m2.
The results of the sensitivity analyses based on the multivariable model of MI, stroke, and all-cause mortality risks, in relation to changes in fasting glucose, from NFG and IFG, are shown in Additional file 1: Tables S1 and S2. The results were almost similar to the results shown in Fig. 2.
Discussion
In this general population-based, retrospective cohort study with more than 2,000,000 person-years of follow-up, we examined the association between 2-year change in fasting serum glucose and risk of cardiovascular disease and all-cause mortality after a median follow-up of 8 years, after adjusting for cardiovascular covariates. Among individuals with NFG at the first health examination, individuals who shifted to IFG were more, and a shift to DFG after 2 years was associated with much higher risks of stroke and all-cause mortality, compared to persistent NFG. Among individuals with IFG at the first health examination, compared to persistent IFG, individuals who shifted to DFG had higher risks of MI and all-cause mortality.
One study examined the effects of change in fasting glucose over time, which was in line with our results. Fasting glucose variabilities are associated with subsequent risks of MI in non-diabetic patients [19]. Other previous studies were also consistent with our findings, although they did not report a change in fasting glucose but rather one-time fasting glucose level. AusDiab study reported that all-cause mortality was greater for IFG than for NFG among 10,428 participants after a median follow-up period of 5.2 years [11]. Additionally, two meta-analyses showed that elevated fasting glucose was associated with the risk of cardiovascular disease in people without diabetes [12, 13]. A prospective study of a large cohort in Korea reported that IFG is associated with the risk of stroke and coronary heart disease [20]. Another prospective national survey in Israel reported that a linear association between admission blood glucose and 10-year mortality among heart failure patients without diabetes [21].
By contrast, some have reported that IFG is less likely to be a risk factor for cardiovascular disease compared to IGT [7,8,9,10]. The differences between the findings of these prior studies and the present study could originate from differences in sample size or from the use of different methods of fasting glucose analysis. Data from several different centers in different countries demonstrate that there is no uniform method for examining fasting glucose [8,9,10], which can result in the possibility of misclassification of fasting glucose status. However, most single large cohorts such as that mentioned above used a uniform analysis method for evaluating fasting glucose, which led to the indication that an IFG could be a significant predictor for cardiovascular disease risks like our findings.
Compared to persistent IFG, shift to DFG from IFG during 2 years (i.e. more rapid change in fasting glucose compare to shift to persistent IFG) was associated with a higher risk of cardiovascular and all-cause mortality, which could imply that glycemic control could slow or halt the progression of macrovascular complications and all-cause mortality, in line with previous studies [21,22,24]. In Korea, when participants revealed an IFG status in the national health screening program, they received an advisory opinion via a letter from a doctor, including suggestions for lifestyle modifications and a recommendation for follow-up 3–6 months later in nearby clinics. According to this advisory opinion, people with prediabetes status intentionally aim to elicit a reduction in hyperglycemia through lifestyle modification such as healthy diets, exercise, quitting smoking, or abstemious in drinking. Accordingly, early detection of IFG via screening of glycemic status could be one of the strategy to prevent mortality. However, the shift to NFG from IFG is not significantly associated with the risk of cardiovascular disease, which may be due to minor events.
The Cardiovascular Heart Study in the U.S. reported no evidence that prediabetes is associated with subsequent 13-year incident cardiovascular events or mortality in community-dwelling adults aged more than 65 years [25]. In our subgroup analysis of participants older than 65 years, the shift to IFG or DFG from NFG was not also significantly associated with risk of MI or stroke; however, it was associated with all-cause mortality. Other previous studies were consistent with our results. Among hospitalized patients with heart failure without pre-existing diabetes, there was a linear relationship between admission glucose level and 10-year mortality [21]. Although heart failure could be the direct cause of death, higher fasting glucose may be an additional contributor to mortality. The difference between the present study and Cardiovascular Heart Study in U.S. is the presence of an independent variable (i.e. change in fasting glucose vs. one-time measurement of fasting glucose). Accordingly, although a prediabetic status itself was not significant among the elderly, increasing fasting glucose was associated with all-cause mortality.
There are some possible mechanisms reported. Abnormal glucose status disrupts normal endothelial function by oxidative stress [26], protein kinase C activation, and advanced glycated end product receptor activation [27], thereby accelerates atherosclerotic plaque formation as well as increasing arterial stiffness [28]. IFG status has also been associated with arterial endothelial dysfunction and intima-media thickening [29], which linked to incident MI and stroke [30, 31]. Furthermore, a series of experimental studies demonstrated that variability in blood glucose may be more prejudiced to increase cardiovascular disease than constantly high blood glucose [31,32,34].
There are limitations to our study that need to be noted. First, the development of MI and stroke confirmed by hospitalization for 2 days or more for the relevant disease based on ICD-10 codes, which are conceivable to underestimate the actual number of cardiovascular disease cases. However, a previous study showed that identifying cardiovascular disease events via the ICD-10 code has an accuracy higher than 80% [35]. Second, future development of diabetes during follow-up period is hard to consider, despite the fact that the risk of major adverse cardiovascular events is significantly greater in diabetic patients who have a longer illness duration [36]. However, we investigated the 2-year change in fasting glucose, which could distinguish the progression to DFG from NFG or IFG status. Third, participants in this study were older than the middle-aged, and the elderly may already have subclinical cardiovascular disease. However, among patients with heart failure, high glucose level was associated with mortality [21]. In addition, some studies reported that target organ damage precedes the clinical appearance of diabetes [37]. Lastly, fasting glucose was assessed through serum, not plasma as was recommended [5], which induced an error in the serum analyses of 1.15% compared to plasma analyses [38]. This error may generate when the sample is stored at room temperature after drawing blood; however, the error is very low because the NHIS recommends refrigeration of the sample at health screening centers. Despite these limitations, these findings show representative results based on data from a nationwide NHIS database [15].
References
Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546.
Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H. The burden of mortality attributable to diabetes. Diabetes Care. 2005;28(9):2130–5.
Morrish N, Wang SL, Stevens L, Fuller J, Keen H, Group WMS. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(2):S14.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA. Consensus statement by the American association of clinical endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2017 executive summary. Endocr Pract. 2017;23(2):207–38.
Marathe PH, Gao HX, Close KL. American Diabetes Association standards of medical care in diabetes 2017. J Diabetes. 2017;9(4):320–4.
Alatorre CI, Hoogwerf BJ, Deeg MA, Nelson DR, Hunter TM, Ng WT, Rekhter MD. Factors associated with stroke, myocardial infarction, ischemic heart disease, unstable angina, or mortality in patients from real world clinical practice with newly-diagnosed type 2 diabetes and early glycemic control. Curr Med Res Opin. 2018;34(2):337–43.
Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. Funagata Diabetes Study. Diabetes care. 1999;22(6):920–4.
Nakagami T, Group DS. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47(3):385–94.
DECODE Study Group; on behalf of the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397–405.
Ning F, Tuomilehto J, Pyörälä K, Onat A, Söderberg S, Qiao Q, Group DS. Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care. 2010;33(10):2211–6.
Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance. Circulation. 2007;116(2):151–7.
Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22(2):233–40.
Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164(19):2147–55.
Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2008;24(1):63–71.
Seong SC, Kim Y-Y, Park SK, Khang YH, Kim HC, Park JH, Kang H-J, Do C-H, Song J-S, Lee E-J. Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7(9):e016640.
Kim MK, Lee W-Y, Kang J-H, Kang J-H, Kim BT, Kim SM, Kim EM, Suh S-H, Shin HJ, Lee KR. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab. 2014;29(4):405–9.
de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity: a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–9.
Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–94.
Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris-Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40(11):1565–72.
Park C, Guallar E, Linton JA, Lee D-C, Jang Y, Son DK, Han E-J, Baek SJ, Yun YD, Jee SH. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36(7):1988–93.
Zadok OIB, Kornowski R, Goldenberg I, Klempfner R, Toledano Y, Biton Y, Fisman EZ, Tenenbaum A, Golovchiner G, Kadmon E. Admission blood glucose and 10-year mortality among patients with or without pre-existing diabetes mellitus hospitalized with heart failure. Cardiovasc Diabetol. 2017;16(1):102.
Alatorre CI, Hoogwerf BJ, Deeg MA, Nelson DR, Hunter TM, Ng WT, Rekhter MD. Factors associated with stroke, myocardial infarction, ischemic heart disease, unstable angina, or mortality in patients from real world clinical practice with newly-diagnosed type 2 diabetes and early glycemic control. Curr Med Res Opin. 2018;34(2):337–43.
de Abreu LL, Holloway KL, Mohebbi M, Sajjad MA, Kotowicz MA, Pasco JA. All-cause mortality risk in Australian women with impaired fasting glucose and diabetes. J Diabetes Res. 2017;2017:8.
Casanova F, Adingupu D, Adams F, Gooding K, Looker H, Aizawa K, Dove F, Elyas S, Belch J, Gates P. The impact of cardiovascular co-morbidities and duration of diabetes on the association between microvascular function and glycaemic control. Cardiovasc Diabetol. 2017;16(1):114.
Deedwania P, Patel K, Fonarow GC, Desai RV, Zhang Y, Feller MA, Ovalle F, Love TE, Aban IB, Mujib M. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol. 2013;168(4):3616–22.
Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung H-J, Rong Y, Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109(4):520–5.
Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163(11):1306–16.
Van Popele NM, Elizabeth Hak A, Mattace-Raso FU, Bots ML, Van Der Kuip DA, Reneman RS, Hoeks AP, Hofman A, Grobbee DE, Witteman J. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: the Rotterdam Study. J Am Geriatr Soc. 2006;54(3):397–404.
Thomas GN, Chook P, Qiao M, Huang XS, Leong HC, Celermajer DS, Woo KS. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. Arterioscler Thromb Vasc Biol. 2004;24(4):739–43.
Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju PM, Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. Stroke. 1995;26(3):386–91.
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340(1):14–22.
Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabo C, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly (ADP-ribose) polymerase. J Thromb Haemost. 2004;2(8):1453–9.
Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281(5):E924–30.
Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells. Diabetes. 2003;52(11):2795–804.
Park JK, Kim KS, Kim CB, Lee TY, Lee KS, Lee DH, Lee S, Jee SH, Suh I, Koh KW. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000;33(1):76–82.
Noh M, Kwon H, Jung CH, Kwon SU, Kim MS, Lee WJ, Park JY, Han Y, Kim H, Kwon T-W. Impact of diabetes duration and degree of carotid artery stenosis on major adverse cardiovascular events: a single-center, retrospective, observational cohort study. Cardiovasc Diabetol. 2017;16(1):74.
Simone G, Wang W, Best LG, Yeh F, Izzo R, Mancusi C, Roman MJ, Lee ET, Howard BV, Devereux RB. Target organ damage and incident type 2 diabetes mellitus: the Strong Heart Study. Cardiovasc Diabetol. 2017;16(1):64.
Frank EA, Shubha M, D’souza CJ. Blood glucose determination: plasma or serum? J Clin Lab Anal. 2012;26(5):317–20.
Authors’ contributions
GL, SMK, and SMP conceptualized the study. SMK conducted the statistical analysis and wrote methods and results parts and GL wrote other sections including abstract, introduction, and discussion as first draft of the manuscript. SC, KK, and JMY did data collection and organizing. JSS and SMJ discussed the results. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Ethics approval and consent to participate
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were waived by the Institutional Review Board of Seoul National University (IRB number: 1703-039-863). All participants were informed regarding the objective of the survey and provided consent. The NHIS database is anonymized according to strict confidentiality guidelines.
Funding
Sung Min Kim and Kyuwoong Kim received a scholarship from the BK21-plus education program provided by the National Research Foundation of the Republic of Korea. This research was supported by Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education (Grant No: 2017R1D1A1B03033721) in the Republic of Korea.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
Associations between change in fasting glucose from the first health examination and the risk of myocardial infarction, stroke, and all-cause mortality excluding participants whose myocardial infarction, stroke, or death occurred in from January 1, 2006 to December 31, 2006. Table S2. Associations between change in fasting glucose at the first health examination and the risk of myocardial infarction, stroke, and all-cause mortality excluding participants whose myocardial infarction, stroke, or death occurred in from January 1, 2006 to December 31, 2007.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, G., Kim, S.M., Choi, S. et al. The effect of change in fasting glucose on the risk of myocardial infarction, stroke, and all-cause mortality: a nationwide cohort study. Cardiovasc Diabetol 17, 51 (2018). https://doi.org/10.1186/s12933-018-0694-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-018-0694-z