Abstract
Background
While, lost to follow-up (LTFU) from antiretroviral therapy (ART) can be considered a catch-all category for patients who miss scheduled visits or medication pick-ups, operational definitions and methods for defining LTFU vary making comparisons across programs challenging. Using weekly cut-offs, we sought to determine the probability that an individual would return to clinic given that they had not yet returned in order to identify the LTFU cut-off that could be used to inform clinical management and tracing procedures.
Methods
Individuals who initiated ART with Dignitas International supported sites (n = 22) in Zomba, Malawi between January 1 2007-June 30 2010 and were ≥ 1 week late for a follow-up visit were included. Lateness was categorized using weekly cut-offs from ≥1 to ≥26 weeks late. At each weekly cut-off, the proportion of patients who returned for a subsequent follow-up visit were identified. Cumulative Distribution Functions (CDFs) were plotted to determine the probability of returning as a function of lateness. Hazard functions were plotted to demonstrate the proportion of patients who returned each weekly interval relative to those who had yet to return.
Results
In total, n = 4484 patients with n = 7316 follow-up visits were included. The number of included follow-up visits per patient ranged from 1–10 (median: 1). Both the CDF and hazard function demonstrated that after being ≥9 weeks late, the proportion of new patients who returned relative to those who had yet to return decreased substantially.
Conclusions
We identified a LTFU definition useful for clinical management. The simple functions plotted here did not require advanced statistical expertise and were created using Microsoft Excel, making it a particularly practical method for HIV programs in resource-constrained settings.
Similar content being viewed by others
Background
Operational definitions of Lost to Follow-up (LTFU) from antiretroviral therapy (ART) vary widely across settings, making comparisons across programs challenging [1]. The development and identification of standardized definitions of LTFU can inform cohort analyses [2, 3], program evaluations [4] and tracing mechanisms. While LTFU can be considered a general ‘catch-all’ category for patients who miss scheduled clinic visits or medication pick-ups [5], different definitions of LTFU within ART programs can have a significant impact on LTFU estimates over time [2, 4]. For example, in their 2013 study Shepherd et al., reported that cumulative estimates of LTFU varied widely, ranging from 22 % to 84 %; this variation was primarily dependent on the definition of LTFU that was applied [4]. This, in turn, has significant implications for program planning purposes such as the development of targeted strategies to improve retention.
Current methods to assess LTFU rates have relied largely on fixed time period cohort approaches, primarily through the use of retrospective cohort analyses. Attrition rates are often reported as proportions of patients who meet a specific outcome at various time points since ART initiation (e.g., 6 months, 12 months) [5–7], although definitions of outcome measures over a specified period of follow-up are generally unclear [8]. Importantly, there is no gold standard to measure retention in care, and different measures (e.g., missed visits, frequency of visits) have different advantages and limitations [9–11]. Generally, when such information is reported and available, patients who are known to have died, stopped ART or transferred out are generally excluded in reported LTFU rates. In addition to a general call for enhanced data on losses to follow-up [8], there has also been a push toward developing more evidence-based definitions [3, 12, 13] that minimize the misclassification of patients as LTFU. Related to this is the need for LTFU definitions that can be used for clinical management purposes. A clinical management definition of LTFU may be particularly useful in settings, such as Malawi, where limited resources are available for tracing. Indeed, high costs and a shortage of human resources necessary for finding missing patients have contributed to a backlog of patients needing to be traced in our setting. Identifying patients earlier who are risk of becoming lost and at risk of experiencing poor clinical outcomes has implications for patient retention overall. At the same time, if patients are prematurely classified as lost, limited resources may be used to trace individuals who ultimately will return on their own. Therefore, a LTFU definition specific to clinical management can inform clinicians of the optimum time to initiate the tracing process (in real time) in such a way that both patient and health system outcomes are maximized.
Chi et al. developed an empirical approach to determining a best-estimate definition of LTFU [3]. In their Zambian study, patients were classified as LTFU based on the number of days they were late for their most recent visit. They used weekly thresholds from ≥1 week to ≥26 weeks late. For each cut-off, they looked forward in their dataset to determine the proportion of patients who returned to care within the subsequent year. The cut-off that minimized the misclassification of patients as LTFU was considered the best-estimate definition, at least based on the available data. Receiver operating curves were plotted to demonstrate the cut-off that best determined whether a patient returned to care within the following year (i.e., the cut-off that minimized the misclassification of patients as LTFU). One of the limitations of this study however, was that only the patients’ last visit was included rather than all visits available [3] and thus not all available data was utilized.
Importantly, clinical decisions in resource-constrained settings are often made with incomplete and/or only partial information [14]. An analytic method, therefore, that is able to make use of all available data would be ideal in order to obtain the most complete picture possible. At the same time, LTFU definitions for clinical management purposes should be simple enough to calculate and interpret so that they can be applied by clinicians and program planners with limited statistical expertise and/or access to specific statistical software to make decisions about patient tracing in real time.
A time to event analysis offers one such method to determine the probability of a patient returning for a subsequent visit based on how late they already are for an expected visit. A time to event analysis studies the time it takes for an outcome to occur, i.e., time to death, time to disease [15, 16] or in this case time to a return. A time to event analysis is often called a survival analysis in the biostatistical literature. However, even when the outcome of interest is not death, one can still use this well-developed body of literature to study the time to non-death events. Cumulative Distribution Functions (CDFs), the complement of the survival function, are a fundamental way to define random variables. Essentially, they describe ‘Area Under the Curve’ functions [17, 18]. CDFs have been used and previously applied in multiple epidemiologic studies including those related to spatial analysis and environmental epidemiology [19–21], surveillance and complex models of disease transmission [22, 23], and studies involving clinical decision-making [14, 24–27]. Related to the CDF is the hazard function, which provides a measure of risk and plots the probability of an event occurring at or over a period of time given that the event has not already occurred [15, 28]. The hazard function in the present analysis provides insight regarding the proportion of patients who return each 1-week interval, of those who have yet to return. As such, clinical staff can use this information to determine when tracing should be initiated (e.g., immediately vs. waiting another week).
CDFs and hazard functions have numerous advantages; for example, they represent data visually in a relatively straightforward and intuitive manner [20]. They can make use of all available data, and as a result tend to present distributions as completely as the dataset allows [15, 29, 30]. Since the CDF is 1 minus the survival function, the variance of the CDF is the same as that of the survival function. This can be calculated through standard methods including Greenwood’s formula [31]. One can refer to most standard books on statistical survival analysis (e.g., Cox & Oakes, 1984) [32].
In the analysis reported here, the CDF and the hazard function were both plotted and examined to determine the probability that a patient would return each week, given that they had not yet returned, in order to identify the LTFU cut-off that could best inform clinical management and tracing procedures.
Methods
Dignitas International (DI), a Canadian non-governmental organization, has worked in partnership with the Malawi Ministry of Health (MOH) since 2004 to support delivery of comprehensive HIV/AIDS care in the Zomba District, one of the most densely populated districts in Malawi (population: 670,500). District HIV prevalence is approximately 14.5 % although estimates within the district vary by location and population group [33]. DI supported the Malawi MOH to establish a tertiary referral HIV clinic at Zomba Central Hospital in 2004, and since 2006 has also supported the Zomba District Health Office to integrate HIV-related services into existing primary health services at decentralized health centres throughout the district [34]. As per Malawian MOH guidelines, each patient who starts ART is given a unique treatment unit ART registration number. This number is written on a paper-based patient card called a Master Card and put into the electronic ART register for staff’s use. All baseline registration data is entered at the time of ART initiation. At each follow-up (FU) visit, patient data is documented on the Master Card. Following initiation, patients are asked to return 2 weeks later and then monthly for the first 6 months. After 6 months, patients may be asked to return every 2–3 months depending primarily upon provider assessments and drug availability [34, 35]. Patients are therefore given a 2-week, 4-week, 8-week or 12-week supply of ART depending on their expected frequency of visits. Data collection on MOH standardized registers and Master Cards are completed by clinicians and health staff at baseline initiation and at each subsequent FU visit.
For each follow-up visit, a ‘days late’ value was determined. The expected return date was calculated by using the value for ART supply (in weeks) given at each FU visit. A patient’s actual return date was compared to their expected return date. A patient was considered late if they did not return at least 7 days after they were expected for a FU visit. This definition is consistent with the 7-days late value used to generate adherence proportions in the Malawi treatment guidelines [35]. As well, discussion with the clinical team in Zomba (personal communication Gabriel Mateyu and Dr. Kevin Bezanson, Dignitas International, Zomba Malawi November 29, 2012) supported use of this definition of Late.
Building on the work of Chi et al. described above [3], we sought to determine a clinical management LTFU cut-off. Individuals 15 years of age and over who initiated ART with Dignitas International (DI) supported sites including the Central hospital and various health centres in rural areas in Zomba, Malawi between 1 January 2007 and 1 July 2010 were eligible for inclusion. The analysis was limited to all follow-up visits where the patient was ≥1 week late. Similar to Chi et al., lateness was categorized using 1-week cut-offs from ≥1 week to ≥26 weeks late [3]. Therefore, this analysis was based on patients who were at least ≥1 week late and likely to have run out of ART, and thus at a high risk of becoming LTFU. Further, patients were excluded if they were known to have transferred out, stopped ART or died, based on the limited successful tracing completed [36]. Note we considered including known deaths as a competing event although we did not feel confident that we could accurately ascertain deaths given incomplete tracing data. Furthermore, only 70 deaths ascertained through tracing were available for this analyses and the median time from a last known visit date and death ascertainment date (through tracing) was 299.5 days. As in Chi et al., we limited our analyses to patients who started ART and had a FU visit scheduled at least 12 months prior to the end of the study period [3]. This helped to ensure that patients had adequate time to return to the clinic [37]. Furthermore, given that the risk of death is often highest in the first few months of treatment [38–41], allowed us to minimize the inclusion of patients who did not return to the clinic because they had died. As we could not ascertain deaths in our dataset, we may have overestimated LTFU estimates.
The DI main dataset, available and maintained in Microsoft ACCESS, is composed of 21 separate tables. Several queries were run in order to construct a single flat file dataset that contained all relevant data (baseline and FU visits) on patients meeting the inclusion criteria. Similar to previous studies utilizing this method [42] the data was presented over a wide range of cut-offs. At each 1-week cut-off, the proportion of patients who returned for a subsequent follow-up visit was identified along with 95 % confidence intervals. The confidence intervals were constructed using the variance of the survival function. Plots were generated in Microsoft Excel 2007 (See Additional files 1 and 2). The data used in this study was extracted from routine monitoring and evaluation data gathered as part of the DI/Malawi MOH ART program in Zomba, Malawi, using standardized national Master Cards and registers. As all analyses were performed with de-identified data that was extracted from routine programmatic information, patients did not provide individual written or verbal consent to participate in the study. Ethical approval for data use was obtained from the University of Toronto HIV Research Ethics Board and the National Health Sciences Research Committee in Malawi.
Results
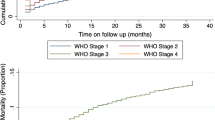
In total, n = 4484 patients with n = 7316 follow-up visits were included. The number of follow-up visits included per patient ranged from 1 to 10 (Median: 1 visit). The median time on ART was 182 days (Interquartile Range: 70–336 days). Included patients were receiving care at n = 22 DI-supported sites. Approximately 40 % of patients were receiving ART at the Central Hospital (n = 1796) and 1929 (i.e., 43 %) were at least 9 weeks late for a visit. The CDF demonstrated that the majority of patients who were ≥1 week late and subsequently returned to care did so within the first 4 weeks of a missed visit, as per the initial sharp increase of the curve (see Fig. 1).
Although fewer, patients who were ≥1 week late continued to return even after being 4 weeks late as demonstrated by the more gradual increase in the curve until approximately the 9-weeks-late cut-off. The curve then begins to flatten out, suggesting that the proportion of patients who returned for a subsequent visit decreased once a patient was approximately 9 weeks late for a visit. The hazard function (Fig. 2) shows two main spikes in the proportion of patients who returned relative to those who had yet to return, roughly at 4-week intervals, at 4 weeks and again around the 8-weeks-late cut-off. As in the CDF, Fig. 2 demonstrates that after being at least 9 weeks late, the proportion of new patients who returned relative to those who had yet to return decreased substantially.
Discussion
The clinical management LTFU definition of approximately 9 weeks identified in this study is relatively consistent with previous literature on losses to follow-up [3, 43, 44]. Chi et al. found that the use of an ≥8-weeks-late cut-off minimized the misclassification of patients as LTFU in their multi-site study in Zambia [3]. The LTFU cut-off in this study is also similar to what is currently used in Malawi to define patients who are LTFU (i.e., 2 months late for a scheduled visit) and this has implications for clinical management guidelines. At the time of the study (i.e., 2007–2010), the Malawian MOH guidelines indicated that home visits (following unsuccessful telephone call attempts) should occur no later than 14 days or 2 weeks after a missed visit [35]. In a parallel, exploratory analysis of Dignitas tracing data, among the 83 patients who were successfully traced (of the 232 with available data), the median number of days from the last visit to a successful trace date was 181.5 days, ranging from 159 days to 306.5 days, considerably longer than the guidelines at the time of this study [36]. Interestingly, the updated 2011 guidelines suggest that home visit attempts should be made from 2 weeks after a missed visit [45]. This subtle change in wording provides patients with more time to return and can thus reduce unnecessary tracing. Indeed, the functions presented indicate that most patients who are already 1 week late and do eventually return for a subsequent visit will return within the first 4 weeks of a missed visit. Currently, limited financial and human resources available for tracing at Dignitas ART sites has led to a backlog of patients waiting to be traced. The findings presented here suggest that in some cases, patients are being traced too prematurely following a missed visit.
While our study identified a LTFU definition for clinical management that made sense in our setting given the data and frequency of visits, it is important to note that the actual definition that we determined (i.e., 9 weeks) may not be appropriate or relevant in other programs and contexts [4]. Indeed, a universal definition or a gold standard measure applied across programs may not be appropriate given different program characteristics [4]. The present study demonstrates LTFU questions can be reframed as a time to event analysis. By reframing the question in this way and by using a well-known, simple, robust, nonparametric technique from this literature, we have found a strong and surprising result that has not, too our knowledge, been reported to date. In the future, we hope to continue modifying techniques from that literature in order to further understand LTFU issues. This would include using methods such as Cox proportional hazard models [46] or Poisson regression methods for life table analysis [47, 48]. Our goal was to introduce a simple methodology based on survival functions that can be used to estimate evidence-based cut-off for defining LTFU specific to clinical management. Investigators interested in examining factors related to lost to follow-up could consider the vast the well-developed literature and additional methods including survival models, Cox proportional hazards models and Poisson regression models. Furthermore, several standardized definitions may be useful depending on the population of interest [4, 11]. For example, a different definition for pre-ART patients may be warranted given different visit frequencies (e.g., no regular pharmacy visits). As a result, a longer LTFU definition may be more appropriate for pre-ART patients [12]. Definitions of LTFU relevant for different population groups should be explored further as they may have different cut-offs that are appropriate.
There are several limitations of using CDFs and hazard functions to establish clinical management LTFU cut-offs. The primary issue relates to the judgement involved in deciding which cut-off is meaningful for categorizing LTFU for clinical management purposes given available data. As noted above, different definitions of LTFU can have a significant impact on LTFU estimates [2, 4]. While the shape of the functions provides some insight (e.g., where the curve flattens in the CDF, spikes in the hazard function), the weeks-late value used to define LTFU in the present study is still a judgement made without a formal set of criteria. The spikes, for example, in the hazard function may be reflective of the expected frequency of visits and the ART supply given (e.g., 4 or 8 week supply). It is worth noting, however, that the visual appraisal of both functions suggests a ≥9-weeks-late clinical management LTFU cut-off. This method therefore has strength in that it can act as a tool for triaging [37] patients for active tracing.
As in other methods, data preparation is a necessary step to ensure that the curves can be generated (See Additional file 1). To establish whether a patient was late for a visit, the ART supply given (in weeks) at their most recent visit was used to determine when they would be expected for their next visit. This information may not always be available given the lack of tracking data and, therefore, the date of the next scheduled visit may also not be reported. Interestingly, 98 % of ART clinics in one East African multi-site study did report recording the next expected appointment date, although under a third actually compared the expected appointment date with the actual return date [1].
Missing data is often a challenge in clinical databases. Generally, patients are said to be censored when information on time to event (of interest) is not available for all participants, including those whom become LTFU [49]. As a result, LTFU is often considered a non-informative censoring event in cohort analyses [2, 50]. In this program of research, however, LTFU is the primary outcome of interest. The reasons for censoring the examined data therefore, are mostly not available, as incomplete follow-up occurs for many patients without adequate resources to enable full or complete follow-up. While death is a competing risk for becoming LTFU (i.e., patients who die are no longer at risk for becoming lost), given incomplete data on known deaths (primarily due to poor ascertainment through tracing) for individuals who had missed their visits, we did not explore death as a competing risk in the present analysis. This is an important limitation as this can lead to overestimations of LTFU [51] even those used for clinical management purposes. Death reporting is neither compulsory nor enforced legally in Malawi [52]. As a result, deaths are generally under reported; this makes linkages with health surveillance and clinical tracking data challenging. However, it is worth noting that while some of those lost to follow-up probably died (about one-third of those successfully traced in this program) effectively taking them out of the denominator of those ‘at risk’ of returning for an additional follow-up visit. However, even if 20 % of those LTFU (larger than the 17 % in Malawi overall) are removed from the denominator i.e. censored, they are unlikely to affect the fundamental shape of the curves substantially, as per the stratified analyses. Indeed, numerous studies have demonstrated higher rates of mortality in the first few months after ART initiation [38–41]. However, while we could not fully account for the impact of deaths in this study, we sought to minimize the number of patients who died in this dataset by excluding patients who had not initiated ART at least 12 months prior to the end of the study although it is important to note that each patient in this study had at least 12 months to return to FU visit as in Chi et al. [12]. As we did not have a specific time limit that patients had to return by in order to be defined as clinically LTFU, there are variable times to return. For example, a patient with a scheduled follow-up visit early in the study period of interest has a longer opportunity to return versus a patients who scheduled FU visit was approximately 12 months from study endpoint (i.e., June 30 2009). Furthermore, a patient may still return after the study period and therefore only have experienced a gap in care rather than be truly LTFU. As visit-level data (versus patient-level) was used to establish clinical management LTFU cut-offs, there may be a differential contribution to data from different patients, as they may have different numbers of follow-up visits included. While it is worth noting that the median number of follow-up visits was 1, the number of follow-up visits ranged from 1 to 10. The small number of follow-up visits per patient may stem from our inclusion criteria of being at least 7 days late for an expected follow-up visit. In a previous analysis [53], we noted that a patient returns to the clinic within 6 days of an expected follow-up visit and are only 7 days late in approximately 17 % of follow-up visits. This may partially explain why the median number of follow-up visits per patient was 1. Regardless, we did not account for multiple follow-up visits per patient in the present study as we were focusing on clinician’s decision about a particular follow-up visit.
CDFs provided a comprehensive presentation of data over a large range of cut-offs and offered guidance regarding the clinical management LTFU cut-off in this setting. The cut-off was further corroborated through the plotting of the associated hazard function. Importantly, there is no gold standard definition of retention and our clinical management LTFU definition may not be appropriate in other settings given that program characteristics and populations of interests can widely across programs and settings [4]. However, having a sense of the proportion of patients who return weekly can help clinical staff, regardless of the program or setting, to predict the number of patients to be expected each week. For example, at the 3 weeks late cut-off, it is expected that approximately 40 % of the remaining patients who are late and who will return will show up during the following week. Clinical staff can then decide the value in waiting another week to start tracing versus implementing tracing immediately.
Conclusions
Flexible and informative, the simple functions plotted here did not require advanced statistical expertise and were created using Microsoft Excel, making it a particularly practical method for HIV programs in resource-constrained settings. As a result, they should be considered additional tools for ART program monitoring specialists and clinical managers in Malawi and other resource-constrained settings. In addition to identifying other clinical management definitions that may be relevant for different population groups (e.g., pre-ART patients), future studies should pilot the use of survival functions and explore the acceptability of this tool among program managers as well as determine their general utility.
Consent for publication
Not applicable.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article (and its additional file).
Abbreviations
- ART:
-
antiretroviral therapy
- CDF:
-
cumulative distribution function
- DI:
-
dignitas international
- FU:
-
follow-up (visit)
- LTFU:
-
lost to follow-up
- MOH:
-
ministry of health (Malawi)
- WHO:
-
World Health Organization
References
Chalker J, Andualem T, Minzi O, Ntaganira J, Ojoo A, Waako P, Ross-Degnan D. Monitoring Adherence and Defaulting for Antiretroviral Therapy in 5 East African Countries: An Urgent Need for Standards. J Int Assoc Physic AIDS Care. 2009;7:193–9.
Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions on loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol. 2013;66:1006–13.
Chi BH, Cantrell RA, Mwango A, Westfall AO, Mutale W, Limbada M, Mulenga LB, Vermund SH, Stringer JS. An empirical approach to defining loss to follow-up among patient enrolled in antiretroviral treatment programs. Am J Epidemiol. 2010;171:924–31.
Shepherd BE, Blevins M, Vaz LME, Moon TD, Kipp AM, Jose E, Ferreira FG, Vermund SH. Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. Am J Epidemiol. 2013;178:819–28.
Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298.
Lamb MR, El-Sadr W, Geng E, Nash D. Association of adherence support and outreach services with total attrition, losses to follow-up, and death among ART patients in sub-Saharan Africa. PLoS One. 2012;7:e38443.
Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 Suppl 1:1–15.
Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056.
Yehia BR, Fleishman JA, Metlay JP, Korthuis PT, Agwu AL, Berry SA, Moore RD, Gebo KA. HIV Research Network: Comparing different measures of retention in outpatient HIV care. AIDS. 2012;26:1131–9.
Chesney MA. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43:S149–55.
Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDs. 2010;24:607–13.
Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, Brinkhof MW, Mwango A, Balestre E, Carriquiry G, Sirisanthana T, Mukumbi H, Martin JN, Grimsrud A, Bacon M, Thiebaut R. International Epidemiologic Databases to Evaluate AIDS Collaboration:. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;9:e1001111.
Forster M, Bailey C, Brinkhof MW, Graber C, Boulle A, Spohr M, Balestre E, May M, Keiser O, Jahn A, Egger M. ART-LINC collaboration of International Epidemiological Databases to Evaluate AIDS: Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ. 2008;86:939–47.
Tom E, Schulman KA. Mathematical models in decision analysis. Infect Control Hospital Epidemiol. 1997;18:65–73.
Kleinbaum DG, Klein M. Survival analysis: a self-learning text. 2nd ed. New York, NY: Springer Science + Business Media, Inc; 2005.
Singh R, Mukopadhyay K. Survival analysis in clinical trials: basics and must know areas. Persp Clin Res. 2011;2:145–8.
Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: John Wiley & Sons; 2004.
Hosmer DW, Lemeshow S. Applied survival analysis: regression modeling of time to event data. New York, NY: John Wiley; 1999.
Zandbergen PA, Chakraborty J. Improving environmental exposure analysis using cumulative distribution functions and individual geocoding. Int J Health Geogr. 2006;5:23.
Dolinoy DC, Miranda ML. GIS modeling of air toxic releases from TRI-reporting and non-TRI reporting facilities: impacts for environmental justice. Env Health Perspectives. 2004;112:1717–24.
Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6.
Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Abu-Raddad LJ, Ho LM, Thach TQ, Chau P, Chan KP, Lam TM, Tsang T, Liu SH, Kong JH, Lau EM, Ferguson NM, Anderson RM. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–6.
Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev. 1994;62:229–43.
Wilson DP, Kahn J, Blower SM. Predicting the epidemiological impact of antiretroviral allocation strategies in KwaZulu-Natal: the effect of the urban divide. Proc Natl Acad Sci U S A. 2011;103:14228–33.
Klotche J, Ferger D, Pieper L, Rehm J. HU Wittchen: oA novel nonparametric approach for estimating cut-offs in continuous risk indicators with application to diabetes epidemiology. BMC Med Res Methodol. 2009;9:63.
Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, Thomson R, Barratt A, Barry M, Bernstein S, Butow P, Clarke A, Entwistle V, Feldman-Stewart D, Holmes-Rovner M,Llewellyn-Thomas H, Moumjid N, Mulley A, Ruland C, Sepucha K, Sykes A, Whelan T. International Patient Decision Aids Standards (IPDAS) Collaboration: Developing a quality criteria framework for patient decision-aids: online international Delphi consensus process. Brit Med J. 2006;333:417.
Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small cell lung cancer: how much benefit is enough? J Clin Oncol. 1993;11:1866–72.
Szklo M, Nieto J. Epidemiology: Beyond the Basics. 2nd ed. Burlington, MA: Jones & Bartlett Learning; 2006.
Papoulis A, Pillai SU. Probability, random variables and stochastic processes. New York, NY: McGraw Hill; 2001.
Tufte ER. The visual display of quantitative information. Cheshire, CT: Graphic Press; 1983.
Greenwood M. The errors of sampling of the survivorship tables, in Reports on the Public Health and Statistical Subjects, no 33. London, UK: HMSO; 1926.
Cox DR, Oakes D. Analysis of survival data. London, UK: Chapman and Hall; 1984.
Malawi National Statistical Office. Malawi Demographic and Health Survey 2010. Zomba, Malawi: National Statistical Office and ORC Macro; 2010.
Chan AK, Mateyu G, Jahn A, Schouten E, Arora P, Mlotha W, Kambanji M, van Lettow M. Outcome assessment of decentralization of antiretroviral therapy provision in a rural of Malawi using an integrated primary care model. Trop Med Int Health. 2010;15 Suppl 1:90–9741.
Government of Malawi Ministry of Health. Treatment of AIDS: Guidelines for the use of antiretroviral therapy in Malawi. 3rd ed. Lilongwe, Malawi: Government of Malawi; 2008.
Rachlis B. Losses to follow-up from an antiretroviral therapy (ART) program in the Zomba District of Malawi [Dissertation]. Toronto: University of Toronto Press; 2013.
Chi BH, Cantrell RA, Zulu I, Mulenga LB, Levy JW, Tambatamba BC, Reid S, Mwango A, Mwinga A, Bulterys M, Saaq MS, Stringer JS. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. Int J Epidemiol. 2009;38:746–56.
Ahonkhai AA, Noubary F, Munro A, Stark R, Wilke M, Freedberg KA, Wood R, Losina E. Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PLoS One. 2012;7:e32993.
Biadgilign S, Reda AA, Digaffe T. Predictors of mortality among HIV infected patients taking antiretroviraltreatment in Ethiopia: a retrospective cohort study. AIDS Res Ther. 2012;9:15.
Coetzee D, Hilderbrand K, Boulle A, Maartens G, Louis F, Labatala V, Reuter H, Ntwana N, Goemaere E. Outcomes after two years of providing antiretroviral treatment in Khayelitsha. South Africa AIDS. 2004;18:887–95.
Weigel R, Estill J, Egger M, Harries AD, Makombe S, Tweya H, Jahn A, Keiser O. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS. 2012;26:365–73.
Farrar JT, Dworkin RH, Max MB. Use of the cumulative proportion of responder analysis graph to present pain data over a range of cut-off points: making clinical trial data more understandable. J Pain Symptom Manag. 2006;31:369–77.
Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Medecins Sans Frontieres: Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS. 2006;20:1163–9.
Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, Karungi G, Szumilin E, Baladine S, Fedida G, Carrieri MP, Spire B, Ford N, Tassie JM, Guerin PJ, Brasher C. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–42.
Government of Malawi Ministry of Health. Clinical management of HIV in children and adults. Lilongwe, Malawi: Government of Malawi; 2011.
Cox DR. Regression Models and Life-Tables. J Royal Stat Soc B. 1972;34:187–220.
Holford TR. The Analysis of Rates and Survivalship Using Log Linear Models. Biometrics. 1980;36:299–305.
Laird N, Olivier D. Covariance Analysis of Censored Survival Data Using Log Linear Analysis Techniques. J Am Stat Assoc. 1981;76:231–40.
Leung KM, Elashoff RM, Afifi AA. Censoring issues in survival analysis. Ann Rev Public Health. 1997;18:83–104.
Greenland S. Application of stratified analysis methods: basic survival analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
Schöni Affolter F, Keiser O, Mwango A, Stringer J, Ledergerber B, Mulenga L, Bucher HC, Westfall AO, Calmy A,Boulle A, Chintu N, Egger M, Chi BH. Swiss HIV Cohort Study; IeDEA Southern Africa: Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: the effect of the competing risk of death in Zambia and Switzerland. PLoS One. 2011;6:e27919.
Zachariah R, Mwagomba B, Misinde D, Mandere BC, Bemeyani A, Ginindza T, Cortier H, Bissel K, Jahn A, Harries AD. Vital registration in rural Africa: is there a way forward to respond to health targets of the Millennium Development Goals? Trans Royal Soc Trop Med Hygiene. 2011;105:301–9.
Rachlis B, Cole DC, van Lettow M, Escobar M, Muula A, Ahmad F, Orbinski J, Chan AK. Follow-up visit patterns in an antiretroviral therapy (ART) programme in Zomba. Malawi PLoS One. 2014;9:e101875.
Acknowledgments
The authors would like to acknowledge the following individuals from Dignitas International for their assistance: Gabriel Mateyu, Dr. Kevin Bezanson, Jean Bourgeois, Alfred Matengeni, and Collins Chisi.
Funding
Personal support for Beth Rachlis was made possible through the Canadian Institute for Health Research, Centre for International Governance Innovation, and the University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
BR conceived the study, conducted the analyses, interpreted the findings and generated a first draft. DCC conceived the study, providing insight regarding analyses and contributed to the drafting of the manuscript. MvL provided context related to Malawi and helped to draft the manuscript. ME conceived the analyses, assisted with the analyses and interpretation and contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1:
Instructions for Using Survival Functions to Generate LTFU Cut-Offs. (DOC 26 kb)
Additional file 2:
Excel Spreadsheet Demonstrating Calculations of Hazard and Cumulative Distribution Functions. (DOC 69 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rachlis, B., Cole, D.C., van Lettow, M. et al. Survival functions for defining a clinical management Lost To Follow-Up (LTFU) cut-off in Antiretroviral Therapy (ART) program in Zomba, Malawi. BMC Med Inform Decis Mak 16, 52 (2016). https://doi.org/10.1186/s12911-016-0290-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-016-0290-7