Abstract
Background
Tuberculosis (TB) kills one child every 5 min. Childhood TB is given low priority in most national health programmes particularly in TB-endemic areas. TB among children is an indicator of a recent transmission of the disease in the community. Treatment outcome results serve as a proxy of the quality of treatment provided by a health care system. In Ethiopia, data on treatment outcomes of childhood TB are limited. The aim of the study was to determine the treatment outcomes of childhood TB in a hospital setting in Addis Ababa.
Methods
The study was conducted during June to August 2014. The data of 491 children treated for TB in Zewditu Memorial Hospital during a 5 year (2009–2013) was analysed. TB was diagnosed using standard methods. Demographic and clinical data including type of TB, TB-HIV co-infection and treatment outcomes were collected from registry of the TB clinic. Treatment outcome definitions are used according to the World Health Organization.
Results
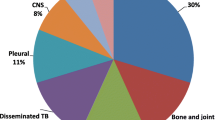
Of the 491 children, 272(55.4 %) were females, 107(21.8 %) were under 5 year old, 454(92.5 %) of them were new cases. The types of TB were extra-pulmonary tuberculosis (EPTB) 243(49.5 %) and 248(50.5 %) pulmonary tuberculosis (PTB). Of the PTB cases, 42(16.9 %) were sputum smear positive. Of the 291 children tested for HIV, 82(28.2 %) were positive. The overall treatment success rate was 420(85.5 %) and the poor treatment outcome was 71(14.5 %). Of the children with poor treatment outcome, 9(1.8 %) died, 3(0.6 %) defaulted from treatment, 2(0.4 %) were treatment failure and 55(11.2 %) were transferred out. Males and females had similar treatment success rates of 85.8 % and 85.3 %, respectively. Infants under one year had significantly lower treatment success rate of 72.7 % compared to those above 1 years of age of 86.5 % (P < 0.001). Treatment success rate ranged from 78.0 to 92.6 % during the study period. Associated factors for treatment outcome were age above 5 years (AOR = 0.59, 95 % CI: 0.62–0.97) and seropositive for HIV infection (AOR = 6.66, 95 % CI: 3.07–14.47).
Conclusions
The treatment success rate in this study is 85.5 %. The outcome of treatment varied with age, and presence of HIV infection. In order to the further improve of treatment success rate, continuous follow up with frequent support of patients during treatment course and strengthen the recording system are strongly recommend.
Similar content being viewed by others
Background
Tuberculosis (TB) remains a major global health problem. It is the second leading cause of death from an infectious disease worldwide, after the human immunodeficiency virus (HIV) [1]. Children account for 6 %–10 % of all TB cases worldwide [2]. TB kills one child every 5 minutes. In countries with high burden TB, it may be as high as 20–40 % of all new TB cases. More than 74,000 children die from the disease each year [2]. Of the one million estimated cases of TB in children worldwide, 75 % occur in the 22 high-burden countries [3]. As childhood TB reflects ongoing transmission in the community, children are affected most acutely in areas where adult TB is poorly controlled [4]. Childhood TB is usually acquired from an infectious adult contact [5]. The global TB control strategy has focused predominantly on smear-positive adult cases and not on the usually paucibacillary and smear negative, noncontagious, asymptomatic childhood TB [6]. TB among children is much more prevalent in developing countries due to poor socio-economic conditions, malnutrition, over-crowding, HIV co-infection and high prevalence of TB in adults contacts [7]. Childhood TB remains neglected for various reasons, mainly the difficulty in diagnosing pulmonary tuberculosis (PTB), the unknown outcomes of children with TB, and the belief that childhood TB is not important for TB control [8, 9]. In 2013, the World Health Organization (WHO) developed a roadmap aiming to achieve zero deaths due to childhood TB by 2025 [10].
Treatment outcome results serve as a proxy of the quality of TB treatment provided by a health care system. Treatment success measured by treatment outcome monitoring (TOM) is a key output of any TB control programs [11]. Treatment outcome in all patients should be routinely monitored by the epidemiological surveillance system. This would make it possible to recognize and amend system failures before the incidence and proportion of resistant isolates rise [12]. According to the 2012 WHO report, Ethiopia ranks seventh among the world’s 22 countries with a high TB burden [1]. Hospital data show that TB is the leading cause of morbidity, third cause of hospital admissions after deliveries and malaria, and second cause of death after malaria in Ethiopia [13]. Although scanty reports on treatment outcomes of TB among adults are available, little is known in children in from Ethiopia [14]. Therefore, this study aimed to assess prevalence and treatment outcomes of TB among children on treatment follow up in Zewditu Memorial Hospital, Addis Ababa.
Methods
Study design, study site and period
A 5-year (2009–2013) hospital- based retrospective cohort study was done to assess the treatment outcomes of childhood TB in Zewditu Memorial Hospital in Addis Ababa. The hospital is one of the six district hospitals under the Addis Ababa City administration health bureau. It is the largest treatment centre of HIV infection in the country. The hospital receives children and adults referred from the city and all over the country for diagnosis and treatment. Data for the study was extracted from registry of TB cases treated under directly observed treatment- short course (DOTS) strategy during January 1, 2009 to December 31, 2013. The study was conducted during June to August 2014.
Study population
A total of 491 children diagnosed as having TB of all types registered during 2009–2013 had follow up in the TB clinic were included in the study. TB patients with incomplete data were excluded from the study. All subjects below the age of 15 years with complete sociodemographic and clinical data were illegible for the study.
Data collection and diagnostic methods
Data were collected on standardized forms that included demographics (age, sex), clinical history, laboratory and radiographic testing, HIV test results, type and category of TB, treatment outcomes. Diagnosis of TB was made according to the recommendations of the Ministry of Health (MoH) of Ethiopia, which was adopted from the WHO [13]. History of contact with TB patient, signs and symptoms suggestive of TB were taken. Patients presenting with cough lasting for more than 2 weeks smear microscopic examination of sputum for bacteriological confirmation was done. Chest x-ray was also taken. Culture for mycobacteria was done particularly for those suspected to have multi drug resistant TB. Patients with at least two positive smears were considered to have smear-positive pulmonary tuberculosis (SPPTB); those with three negative smears were treated with antibiotics and then re-evaluated. Diagnosis of extra pulmonary tuberculosis (EPTB) was based on fine needle aspiration cytology, biochemical analyses of cerebrospinal/pleural/ ascetic fluids or histopathological examination in addition to clinical evidences consistent with active EPTB. Treatment was based on the the guidelines of the country with a full course of anti-TB therapy (DOTs). Treatment of new TB patients consists of a 2-month intensive phase with Isoniazid, Rifampicin, Pyrazinamide and Ethambutol followed by a 4-month continuation phase with Rifampicin and Isoniazid. Outcomes were categorized as favorable (cured/success) and unfavorable (loss to follow-up, died, failure and transfer out).
Data analysis
Data was entered using Epi-Data version 3.1 and analyzed using computer software SPSS version 20. Descriptive statistical methods were used to generate frequencies of categorical variables and univariate and multivariate logistic regression analysis was used to investigate the effect of selected risk factors on the incidence of treatment success. The univariate (unadjusted) and multivariate (adjusted) logistic regression analysis were used for odds ratios (ORs) and 95 % confidence interval (95 % CI) was done to assess the association between potential risk factors and treatment outcome. P value of 0.05 was used as the cut-off point for statistical significance.
Ethical issues
Ethical clearance was obtained from Department Ethical and Review Committee (DERC) of Microbiology, Immunology and Parasitology (DMIP), College of Health Sciences, Addis Ababa University. Consent was obtained from parents or guardians during examinations in the TB clinic of the hospital. In order to ensure confidentiality, names of study participants were not included in the data sheet. Information obtained from the data of the study participants is kept confidential.
Definitions of terms
TB cases were defined according to WHO criteria Table 1.
Results
Sociodemographic and clinical characteristics of children with tuberculosis
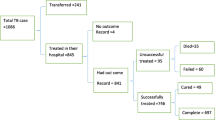
A total of 652 children diagnosed to have TB in Zewditu Memorial Hospital were included in the study. Of these, 161(24.7 %) were excluded because of incomplete data while 491(75.3 %) of the patients treated for different TB types had were illegible and data were analysed. Of the 491children, 219(44.6 %) were males and 272(55.4 %) females with age range from zero to14 years (mean age of 9.0 ± 4.5SD). Of the total children, under 1 year old had the least contribution of 33(6.7 %) of the total. In total, the under five children comprised of 107(21.8 %). Nearly half, 245(49.9 %) were in the age range of 10–14 years. The remaining patients with incomplete data were excluded from the study. Of all the TB cases, 243(49.5 %) were due to EPTB. Of the PTB cases, 206(83.1 %) were SNPTB and 42(16.9 %) were SPPTB cases. Of the Majority of the children, 454(92.5 %) were new cases, while 19(3.9 %) were transferred in, 5(1.0 %) were retreatment (relapse), 3(0.6 %) cases were default and 2(0.4 %) cases were treatment failures. Of the 291 children tested for HIV, 82(28.2 %) of them were positive thus had TB -HIV co-infection (Table 2).
Treatment outcomes
The treatment outcome of the 491 paediatrics TB patients assessed showed that the overall successful treatment (cure rate) was 420(85.5 %). The treatment success in males was 85.8 % while it was 84.9 % in females (P > 0.05). There was no significant differences in the treatment success of new TB cases compared to relapse cases which was 85.7 and 60 %, respectively (P = 0.32). The treatment success of HIV negative children was higher, 93.8 % compared to children with TB-HIV co-infection who had cure rate of 70.7 % (P =0.00). Similarly, children with unknown HIV serostatus had a higher treatment success of 82.5 % compared to the 70.7 % of the HIV positive cases (P = 0.00). Children in the age group 5–9 had higher treatment success of 123(88.5 %) compared to children of under 1 year of age 24(72.7 %) (P < 0.05). The treatment success was higher among patients with EPTB, (86.8 %) compared to those with PTB (81.0 % for SPPTB and 85.0 % of SNPTB).
The poor outcome of treatment identified showed that 3(0.6 %) were defaulters from treatment, 9(1.8 %) died, 2(0.4 %) were treatment failure and 55(11.2 %) were transferred out. The treatment outcome reports were unknown in 3(0.6 %) cases of the pediatric patients. The death rate is higher in the age range of 1–4 which was 3/74(4.1 %) followed by age group 10–14 with death rate of 4/245(1.6 %) (Table 3).
Trend analysis of treatment outcomes in the 5 years
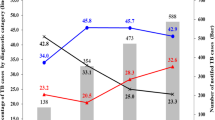
Majority of the study participants, 454(92.8 %) were new cases. The trend of the treatment outcome showed an increment in treatment success rate during the study period ranging from 78.0 % in 2009 to 92.6 % in 2013. The incidence of the disease slightly decreased across the years, except in 2010 which showed increment.
In the study, the rate of transfer out varied from 5(5.1 %) in 2009 to 20 (20.0 %) in 2012. Highest death rate of 4.1 % was observed in 2012 (Table 4).
The proportions of SPPTB was 42 (8.6 %), the highest being 9.9 % during 2010 (Table 4). The trend of EPTB cases varied across the study period from (52.0 %) in 2011 to (48.0 %) in 2012. The trend of unsatisfactory treatment outcomes during the study period (i.e. died, failed, defaulted and unknown treatment outcome) is presented in (Fig. 1). The assessment of TB types in the 5 years showed that the percent of EPTB cases ranged from 48 to 52 % compared to the SPPTB cases which ranged from 7.1 to 9.9 % and SNPTB cases from 38.8 to 47.9 % (Fig. 2). Treatment success rate consistently increased progressively from 78 % during 2009 to 91.4 % in 2013. The highest treatment success rate (TSR) of 91.4 % was observed in 2013 compared to TSRs across the earlier years (Fig. 3).
Treatment outcome and associated factors
There was no significant difference in the treatment outcomes among male and female patients (P = 0.97). Children in the age group 5–9 years had statistically significant treatment success rate compared to the under 1 year age children (P = 0.04). On the other hand in multivariate adjusted model, variables like age, HIV status and pulmonary positive case types have shown significant association with the treatment success (Table 4). On multivariate logistic regression, children in the age group 5–9 years were independently associated with successful treatment outcomes [AOR = 0.59 (0.62–0.97), P = 0.04].
On the other hand, patients with SPPTB [AOR = 0.72 (0.53 – 0.92) and those with unknown serostatus [AOR = 0.94 (0.57 – 1.68)] had significantly lower treatment success rates.
Children who were HIV negative showed higher rate of treatment success compared to the HIV positive patients [AOR = 6.66 (3.07–14.47), p =0.00). The associated factors with TSRs were depicted in Table 5.
Discussion
As childhood TB reflects recent transmission, its burden provides an accurate measure of the level of TB in a community [15]. Treatment outcomes of TB in children are rarely evaluated by most TB programs in sub-Saharan Africa [16]. In 2007, the WHO has called for more studies to define the global epidemiology of childhood TB because the literature remains scant, dominated primarily by studies from industrialized countries [17]. Under 1 year-old children had the least involvement in 33 (6.7 %) while under 5 children constituted 107 (11.4 %) of the total TB patient population. This is similar to a study in India reporting 11 % despite the fact that rates of childhood TB are usually considered the highest among those aged 1–4 years [18]. Under-five children constituted the majority of childhood TB in previous studies in Africa [19] and Thailand [20]. The low number of under-1 year children with TB in this study could be due to missing of cases in investigation because of the diagnostic difficulties. In this study, older children in the age range of 10–14 years represented nearly half (49.9 %) of the cases with TB. This finding is similar to a report by Hailu et al. [14] who reported that 48.4 % of all the children assessed for TB were in the age range of 10–14 years.
TB was slightly higher in female children with male: female ratio of 0.8:1 (P > 0.05). This finding is in agreement with the previous study conducted in India, where high prevalence of 61.7 % was reported in females than in males [21]. However, many studies show similar infection rates in children unlike the predominance in adult males [22].
In the present study, nearly half of the cases, 243 (49.5 %) were due to EPTB, while the remaining 248 (50.5 %) were due to PTB. Of the PTB patients, 42 (16.9 %) had SPPTB and 206 (83.1 %) had SNPTB. The detection rate of M. tuberculosis (smear positivity) was slightly higher than a report by the WHO in which smear-positive TB in children aged <14 years accounted for 0.6–3.6 % [4]. However, our finding of SPPTB is similar with the16.8 % report by Tessema et al. [12]. However, the smear positivity rate is lower than a study done by Alavi et al. (2015) from Iran who reported a high rate of smear positive cases of 73 (72.3 %) [23].
In the present study, there was a clear trend of smear positivity in children with increasing age despite the overall prevalence EPTB children is higher. Majority of the children were <10 years old which is in agreement with previous studies [24]. However, the higher smear positivity was seen in older children. This could be due to the fact that young children are unable to produce sputum and thus have paucibacillary PTB. Thus, they are more likely to have EPTB than older children. In TB- endemic areas, the diagnosis of TB in children at lower age is mostly based on clinical and x-ray examinations. Thus, advanced TB diagnostic tools are critical for the study to avoid missing of childhood TB cases. Majority of the study participants, 454 (92.5 %) were new cases. This shows that the relapse and transferred in cases were small in number. HIV infection increases the susceptibility to TB. In this study, the prevalence of TB-HIV co-infection was 82 (28.2 %). Adejumo et al. [16] from Nigeria reported a 29 % co-infection and 27 % report from Thailand [20]. However, it is higher compared to previous reports of 10.9 % by Beza et al. (2013) from Ethiopia [25]. However, this is lower than a prevalence of 52 % reported by Fairlie et al. (2011) from South Africa [26].
The proportion of children with successful outcome is an indicator of the quality of TB case management [27]. In this study, the overall treatment success rate among all children with TB was 85.5 % which meets the WHO targeted of 85 % first set by the World Health Assembly in 1991 [1]. However, this finding was higher than in previous reports from southern Ethiopia where the treatment success rate was 49 % [28]. The proportions of treatment success were 85.7 % for new and 60 % for relapse (retreatment) cases for the entire study period (P < 0.05). Children under 1 year old had worst treatment outcome of 72.7 %. Similar finding was reported by Adejumo et al. from Nigeria [16]. Children less than 5 years old had a lower treatment success compared to those above 5 years (82.2 % versus 86.5 %, respectively). These are consistent with a study by Dangisso et al. [29].
Although the outcome of TB treatment in children is frequently not reported for various reasons some of the facts include lack of scientific studies, and the belief that childhood TB is not important factor for TB control [7]. In addition, socioeconomic conditions, malnutrition, over-crowding and HIV co-infection also contribute for the lower treatment outcomes [6]. This study found that the treatment outcome of childhood TB treated under DOTs program at Zewditu Memorial Hospital was satisfactory. The relatively higher success rate could be due to better case management with the availability of free TB treatment in the study health facilities. In addition, the low death rate of 1.8 % for all pediatric TB cases is lower than 5.8 % reported from Southern Ethiopia [8], 10.5 % reported from Botswana [30], 10.9 % from Tanzania [19] and 17 % from Malawi [31].
This study showed an overall default rate of 0.2 % among the pediatric TB patients in the study health facilities in the last 5 years. This is much lower compared to results from previous studies which showed high default rate in Ethiopia ranging from 3.8–20 % [32, 33].
Defaulter rate was low compared to a report in rural areas of South Ethiopia which was 13.9 % [34]. The mortality rate of 1.8 %) in our study consistent with a 3.3 % result reported by Hailu et al. [14] but lower than the rate 7.1 % reported by Salarri et al. from Iran [35]. Ethiopia is one of the highly affected countries by the TB/HIV co-epidemic. The WHO global report of 2008 estimated that in Ethiopia 40 % of TB patients tested for HIV were positive, while routine data from 2006/7 estimated that 31 % of TB patients were HIV positive [8]. In the present study, 82 (16.7 %) children had TB/HIV co-infection. This result is lower than the previous reports because of the reduced incidence of HIV infection in the country. Recent studies have reported that co-infection with HIV, young age, having smear-positive PTB, failing to convert to smear-negative after 2 months of treatment and living in a rural area were independent risk factors for unfavorable treatment outcome [7, 26]. Similarly, in the current study, co-infection with HIV, and age group of 5–9 year were risk factors for unfavorable outcome (Additional file 1). The higher incidence of serious forms of TB (such as meningitis or miliary TB), the immaturity of the immune system, delayed diagnosis due to the low sensitivity of diagnostic techniques and the higher prevalence of other adverse conditions such as malnutrition in young children have been reported as factors contributing towards poor treatment outcome at this age [5]. Few years back, Graham and his colleagues [36] reported that Malawian children treated for TB often had insufficient blood levels of medication, which could be due to several reasons including inappropriate drug dosage and lower absorption. Similarly, Young children who are unable, or unwilling to swallow large number of tablets each day contributing to poor treatment outcomes. Thus, child friendly formulations are the most critical need to treat TB [37].
The limitations of the study include many patients were excluded from the study due to incomplete records. Another limitation was the absence of microbiologic confirmation in most diagnosed patients.
Conclusions
TB treatment success rate in the current study has similar to the first set WHO target of 85 % and is higher than that reported from other studies in the country and the region. This relatively higher success rate could be attributable to better case management with the availability of treatment in the study area. This study showed that the outcome of treatment varied with age, sex and type of TB. In order to further improve the treatment success rate, continuous follow up with frequent support of patients during treatment course and strengthen the recording system are strongly recommended.
Abbreviations
aOR, adjusted odds ratio; HIV, human immunodeficiency virus; TB, tuberculosis; WHO, World Health Organization; DOTS, directly observed treatment- short course; MoH, ministry of health; PTB, pulmonary tuberculosis; EPTB, extra pulmonary tuberculosis; SPPTB, smear-positive pulmonary tuberculosis; SNPTB, smear-negative pulmonary tuberculosis
References
WHO. Global Tuberculosis report 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO; 2012.
Hamzaoui A, Yaaloui S, Cherif FT, Saidi ML, Berraies A. Childhood tuberculosis: a concern of modern world. Eur Respir Rev. 2014;23:278–91.
Seth V, Kabra SK. Essentials of tuberculosis in children. 3rd Ed. 2006.
Marais BJ. Childhood tuberculosis: epidemiology and natural history of disease. Indian J Pediatr. 2011;78:321–7.
Centers for Disease Control and Prevention (CDC). Reported Tuberculosis in the United States. 2013. www.cdc.gov/tb/. Accessed Sept 2014.
Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367:348–61.
Marais BJ, Gie RP, Hesseling AC, Schaaf SH, Lombard C, Enarson ED, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics. 2006;118:e1350–9.
Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Pediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510.
Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. Int J Tuberc Lung Dis. 2004;8:636–47.
World Health Organization. Roadmap for Childhood Tuberculosis: Towards Zero Deaths. Geneva: WHO; 2013.
Van Hest R, Kodmon C, Verver S, Erkens CGM, Straetemans M, Manissero D, et al. Tuberculosis treatment outcome monitoring in European Union countries: systematic review. Eur Respir J. 2013;41:635–43.
Tessema B, Muche A, Bekele A, Resing D, Emmrich F, Sack U. Treatment outcome of Tuberculosis patients at Gondar University Teaching Hospital, Northwest Ethiopia: A five -year retrospective Study. BMC Infect Dis. 2009;9:371.
Federal Ministry of Health (FMOH). Tuberculosis, Leprosy and TB/HIV Prevention and Control Programme Manual. 4th ed. Addis Ababa: MOH; 2008.
Hailu D, Abegaz WE, Belay M. Childhood tuberculosis and its treatment outcomes in Addis Ababa: a 5-years retrospective study. BMC Pediatr. 2014;14:61.
Hamzaoui A, Yaalaoui S, Cherif FT, Saidi LS, Berraies A. Childhood tuberculosis: a concern of the modern world. Eur Resp Rev. 2014;23:133.
Adejumo OA, Daniel OJ, Adebayo BI, Adejumo EN, Jaiyesimi EO, Akang G, et al. Treatment Outcomes of Childhood TB in Lagos. Nigeria J Trop Pediatr. 2015. doi:10.1093/tropej/fmv089.
World Health Organization. A research agenda for childhood tuberculosis: improving the management of childhood tuberculosis within national tuberculosis programmes: research priorities based on a literature review. Geneva, Switzerland: WHO; 2007.
Satyanarayana S, Shivashankar R, Vashist RP, Chauhan LS, Chadha SS, Dewan PK, et al. Characteristics and Programme-Defined Treatment Outcomes among Childhood Tuberculosis (TB) Patients under the National TB Programme in Delhi. PLoS One. 2010;5(10):e13338. doi:10.1371/journal.pone.0013338.
Mtabho CM, Irongo CF, Boeree MJ, Aarnoutse RE, Kibiki GS. Childhood Tuberculosis in the Kilimanjaro region: lessons from and for the TB Programme. Trop Med Int Health. 2010;15:496–501.
Lolekha R, Anuwatnonthakate A, Nateniyom S, Sumnapun S, Yamada N, Wattanaamornkiat W, et al. Childhood TB epidemiology and treatment outcomes in Thailand: a TB active surveillance network, 2004 to 2006. BMC Infect Dis. 2008;8:94. doi:10.1186/1471-2334-8-94.
Sharma SR, Sarin R, Khalid UK, Singla N, Sharma PP, Behera D. The DOTS strategy for treatment of pediatric pulmonary tuberculosis in South Delhi, India. Int J Tuberc Lung Dis. 2008;11:74–80.
Sabawoon W, Sato H. Sex Difference in Tuberculosis in Afghanistan: A National Cohort Study. Mycobac Dis. 2012;2:115. doi:10.4172/2161-1068.1000115.
Alavi SM, Salmanzadeh S, Bakhtiyariniya P, Albagi A, Hemmatnia F, Alavi L. Prevalence and treatment outcome of pulmonary and extrapulmonary pediatric tuberculosis in southwestern Iran. Caspian J Intern Med. 2015;6:213–9.
Ramos JM, Reyes F, Facin R, Tesfamariam A. Surgical lymphnode biopsies in a rural Ethiopian hospital: histopathology diagnoses and clinical characteristics. Ethiop Med J. 2008;46:173–8.
Beza MG, Wubie MT, Teferi MD, Getahun YS, Bogale SM, Tefera SB. A Five Years Tuberculosis Treatment Outcome at Kolla Diba Health Center, Dembia District, Northwest Ethiopia: A Retrospective Cross-sectional Analysis. J Infect Dis Ther. 2013;1:101.
Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi SA. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC Infect Dis. 2011;11:28.
WHO. Guidance for national tuberculosis programmes on the management of tuberculosis in children. WHO/HTM/TB/2006.371, WHO/FCH/CAH/2006.7. Geneva, Switzerland: WHO; 2006.
Shargie E, Lindtjørn B. DOTS improves treatment outcomes and service coverage for tuberculosis in South Ethiopia: a retrospective trend analysis. BMC Pub Health. 2005;5:1471–7.
Dangisso MH, Datiko DG, Lindtjorn B. Low case notification rates of childhood tuberculosis in southern Ethiopia. BMC Pediatrics December. 2015;15:142.
Oeltmann JE, Chengeta B, Mboya JJ, Wells CD, Kilmarx PH, Samandari T, et al. Reported childhood tuberculosis treatment outcomes, Gaborone and Francistown, Botswana, 1998-2002. Int J Tuberc Lung Dis. 2008;12:186–92.
Harries AD, Hargreaves NJ, Graham SM, Mwansambo C, Kazembe P, Broadhead RL, et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. In J Tuberc Lung Dis. 2002;6:424–31.
Shargie EB, Lindtjorn B. Determinants of treatment adherence among smear-positive pulmonary tuberculosis patients in southern Ethiopia. PLOS, Feb. 13, 2007 DOI: 10.1371/journal.pmed.0040037
Ejeta E, Chala M, Arega G, Ayalsew K, Tesfaye L, Birhanu T, et al. Outcome of Tuberculosis patients under directly observed short course treatment in western Ethiopia. J Infect Dev Ctries. 2015;9(7):752–9.
Ramos JM, Reyes F, Tesfamariam A. Childhood and adult tuberculosis in a rural hospital in Southeast Ethiopia: a ten-year retrospective study. BMC Public Health. 2010;10:215. doi:10.1186/1471-2458-10-215.
Salarri MH, Kalantari AB. Characteristics of tuberculosis patients in Yazd province, Islamic Republic of Iran, 1997-99. East Mediterr Health J. 2004;10:175–9.
Graham SM. Treatment of pediatric TB: revised WHO guidelines. Pediatr Respir Rev. 2011;12:222–6.
Vijayasekaran D. Treatment of childhood tuberculosis. Indian J Pediatr. 2011;78:443–8.
Acknowledgments
We would like to thank Zewditu Memorial hospital TB clinic staff for their assistance in the data collection process and provision of relevant information for research.
Funding
There is no source of funding.
Availability of data and materials
The raw data are provided here.
Authors’ contributions
GT conceived the study, designed, participated in data collection, conducted data analysis, entered data and interpreted the results. SGT reviewed the initial and final drafts of the paper, and drafted the paper for publication. Both authors read and approved the final paper.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Ethical clearance was obtained from Department Ethical and Review Committee (DERC) of Microbiology, Immunology and Parasitology (DMIP), College of Health Sciences, Addis Ababa University. Consent was obtained from parents or guardians during examinations in the routine TB clinic of the hospital. In order to ensure confidentiality, names of study participants were not included in the data. Information obtained from the data of the study participants is kept confidential.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Childhood TB in Addis Ababa, Data. (XLS 70 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tilahun, G., Gebre-Selassie, S. Treatment outcomes of childhood tuberculosis in Addis Ababa: a five-year retrospective analysis. BMC Public Health 16, 612 (2016). https://doi.org/10.1186/s12889-016-3193-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-016-3193-8