Abstract
Background
Our previous study suggested that the recurrent CHEK2 H371Y mutation is a novel pathogenic mutation that confers an increased risk of breast cancer. The purpose of this study was to investigate whether breast cancer patients with CHEK2 H371Y mutation were more likely to respond to neoadjuvant chemotherapy.
Methods
We screened a cohort of 2334 Chinese women with operable primary breast cancer who received a neoadjuvant chemotherapy regimen for CHEK2 H371Y germline mutations. Pathologic complete response (pCR) was defined as the absence of tumor cells in the breast after the completion of neoadjuvant chemotherapy.
Results
Thirty-nine patients (1.7%) with CHEK2 H371Y germline mutation were identified in this cohort of 2334 patients. CHEK2 H371Y mutation carriers had a significantly higher pCR rate than non-carriers (33.3% versus 19.5%, P = 0.031) in the entire study population, and CHEK2 H371Y mutation-positive status remained an independent favorable predictor of pCR in a multivariate analysis (odds ratio [OR] = 3.01; 95% confidence interval [CI]: 1.34- 6.78, P = 0.008). CHEK2 H371Y carriers had a slightly worse distant recurrence-free survival than non-carriers (adjusted hazard ratio [HR] =1.24, 95% CI: 0.59-2.63).
Conclusions
CHEK2 H371Y mutation carriers are more likely to respond to neoadjuvant chemotherapy than are non-carriers.
Similar content being viewed by others
Background
CHEK2 (Cell-cycle-checkpoint kinase 2, also known as CHK2) encodes a multifunctional kinase that is activated mainly by the ataxia-telangiectasia mutated (ATM) protein in response to DNA double-strand breaks [1-4]. Activated CHEK2 in turn phosphorylates several critical cell-cycle proteins, including p53, Cdc25 and BRCA1, which trigger cell-cycle arrest, apoptosis, and the activation of DNA repair [5-7].
Numerous studies have demonstrated that CHEK2 is a moderate breast cancer susceptibility gene [8-12]. CHEK2 1100delC, a truncating mutation that abrogates the kinase activity of the protein, confers an approximately 2-fold increase in breast cancer risk [8,13-15]. However, the prevalence of CHEK2 1100delC mutation varies widely among ethnic groups [8,11,16-19]. The mutation is mostly found in the Dutch population [8,20], and it is absent or very rare in other populations [16-19]. We previously screened 2255 Chinese women (1027 breast cancer cases and 1228 healthy controls) for CHEK2 1100delC and failed to find this mutation in this population. However, a novel recurrent CHEK2 mutation near the CHEK2 1100delC mutation, CHEK2 1111C > T (H371Y), was found in Chinese women [21]. CHEK2 H371Y is within the activation loop of the CHEK2 protein kinase domain, which is essential for the activation of CHEK2 in response to DNA damage. Functional analysis reveals that the CHEK2 H371Y mutation produces a dramatic decline in CHEK2 activity and is a pathogenic mutation [21]. CHEK2 H371Y confers a 2.43-fold increase in breast cancer risk in Chinese women.
The disruption of CHEK2 kinase activity may not only contribute to breast cancer development but also influence breast cancer survival or response to the adjuvant therapy. Two studies have suggested that the CHEK2 1100delC mutation is associated with poor recurrence-free survival in breast cancer [22,23], indicating that patients with CHEK2 1100delC mutation have an aggressive phenotype.
No previous studies have investigated the association between CHEK2 germline mutation and response to neoadjuvant chemotherapy in breast cancer. Therefore, in the current study, we investigated whether CHEK2 H371Y mutation carriers are more likely to respond to neoadjuvant chemotherapy in terms of pathologic complete response (pCR) in a large cohort of 2334 breast cancer patients who received neoadjuvant chemotherapy and further explored the association between CHEK2 H371Y mutation status and distant recurrence-free survival (DRFS).
Methods
Study population
A total of 2382 operable primary breast cancer patients with stage I-III were treated with neoadjuvant chemotherapy at the Breast Center of Peking University Cancer Hospital from October 2003 to December 2010. The mean age of the subjects was 47.6 years (range, 22–75 years). Tumor stage was classified according to the tumor-node-metastasis classification of the Union Internationale Contre le Cancer. Tumor size was defined as the maximum tumor diameter measured on the mammogram and/or ultrasonogram at the time of diagnosis. The tumors were graded according to the modified Bloom-Richardson system. Written consent was obtained from all subjects. This study was approved by the Research and Ethical Committee of Peking University Cancer Hospital.
CHEK2 H371Y germline mutations
Peripheral blood samples were collected from all patients. Genomic DNA was extracted from the leukocyte pellet by proteinase K digestion followed by phenol-chloroform extraction. The CHEK2 H371Y mutation was detected by polymerase chain reaction (PCR) followed by denaturing high-performance liquid chromatography (DHPLC) and sequencing or directed sequencing as described previously [21]. We screened all 2382 patients for the germline CHEK2 H371Y mutation. CHEK2 H371Y status was not readable for 46 patients, and 41 of the 2336 patients were found to carry the mutation. We then screened these 41 CHEK2 H371Y mutation carriers for germline mutations in BRCA1/2; two patients also carried a BRCA2 germline mutation and were excluded from this study. Therefore, 2334 patients, 39 of whom were CHEK2 H371Y carriers, were included in the final analysis in the current study.
Estrogen receptor (ER), progesterone receptor (PR), and HER2 status
ER, PR, and HER2 status were determined in the core-needle biopsy breast cancer tissue obtained before the initiation of neoadjuvant chemotherapy as described previously [24].
Neoadjuvant chemotherapy regimens
Among the 2334 patients who received neoadjuvant chemotherapy, 94% received 4–8 cycles. Treatments were categorized in three subgroups as follows:
-
(1)
859 patients received an anthracycline-based regimen. The detail of the regimens are described previously [24]. Of these, 537 patients received a CTF regimen; 247 patients received an FEC regimen; 59 patients received a CAF regimen; the remaining 16 patients received other types of anthracycline regimens.
-
(2)
882 patients received an anthracycline-taxane containing regimen. Of these, 682 patients received two cycles of anthracycline followed by 4 cycles of paclitaxel alone (80 mg/m2 IV once per week for 12 weeks) or paclitaxel plus carboplatin (paclitaxel 175 mg/m2 IV on day 1 or paclitaxel 60 mg/m2 IV on day 1, day 8, and day 15, and carboplatin AUC 6 IV on day 1 every three weeks); 181 patients received 4 cycles of paclitaxel alone or docetaxel plus cyclophosphamide (docetaxel 75 mg/m2 IV on day 1 and cyclophosphamide 600 mg/m2 IV on day 1 every three weeks), followed by 2 to 4 cycles of anthracyclines. The remaining 19 patients received other types of anthracycline/taxane containing regimens, i.e., a TE regimen (docetaxel plus epirubicin) or TAC regimen (docetaxel, doxorubicin, and cyclophosphamide).
-
(3)
593 patients received a taxane-based regimen without anthracyclines. Of these, 494 patients received 4 cycles of paclitaxel (80 mg/m2 IV once a week for 12 weeks); 76 patients received paclitaxel plus carboplatin (paclitaxel 60 mg/m2 IV on day 1, day 8, and day 15, and carboplatin AUC 6 IV on day 1 every three weeks). The remaining 23 patients received docetaxel plus cyclophosphamide (docetaxel 75 mg/m2 IV on day 1 and cyclophosphamide 600 mg/m2 IV on day 1 every three weeks).
In this cohort of 2334 patients, 108 patients received intravenous trastuzumab in combination with neoadjuvant chemotherapy.
After completion of neoadjuvant chemotherapy, patients were treated with mastectomy (n = 1351) or breast-conserving surgery (n = 983) depending on the tumor size, presence of multiple lesions or patient preference. pCR was defined as the absence of invasive breast cancer cells in the breast after the completion of neoadjuvant chemotherapy [25,26].
Sixty-two percent of patients received adjuvant chemotherapy with the same or alternative regimens after operation; patients with axially positive lymph nodes and/or breast-conserving therapy received radiotherapy; patients with ER and/or PR-positive disease received endocrine therapy (20 mg/d tamoxifen for 5 years or 1 mg/d anastrozole for 5 years).
Statistical analysis
The differences in clinicopathological characteristics between CHEK2 H371Y carriers and non-carriers were determined by Pearson’s chi-squared test. The associations between CHEK2 H371Y mutation status, clinicopathologic characteristics, and pathological response to neoadjuvant chemotherapy were determined by Pearson’s chi-squared test or Fisher’s exact test when the number of patients was small. A logistic regression model was applied to determine whether a factor was an independent predictor of pCR in a multivariate analysis. Distant recurrence-free survival (DRFS) was defined as the time from the date of diagnosis to first distant recurrence (not including second primary malignancies) or death from breast cancer without a recorded relapse. Survival curves were derived from Kaplan–Meier estimates and compared using log-rank tests. All statistical tests were two-sided, and P values <0.05 were considered statistically significant. The statistical analyses were performed using SPSS 16.0 software (Chicago, IL, USA).
Results
Patient and tumor characteristics
In this cohort of 2334 patients, 39 women carried a CHEK2 H371Y mutation (39/2334, 1.7%). Since the primers used in this study covered the CHEK2 1100delC mutation, none CHEK2 1100delC was found in the current study. The clinicopathological characteristics and chemotherapy regimens of each group are presented in Table 1. No significant differences were found between CHEK2 H371Y carriers and non-carriers with regard to tumor size, lymph node status, ER or PR status, chemotherapy regimens, surgery type, tumor grade, and pathological type (Table 1). However, CHEK2 carriers were more likely to be diagnosed at or before age of 50 as compared with non-carriers (P = 0.036, Table 1), and CHEK2 carriers were less likely to be HER2-positive (15.4%) than non-carriers (30.8%) (P = 0.038, Table 1).
Response to neoadjuvant chemotherapy in CHEK2 carriers and non-carriers
Overall, 460 patients (19.7%) achieved a pCR after neoadjuvant chemotherapy. The pCR rate was 33.3% (13/39) for CHEK2 H371Y mutation carriers and 19.5% (447/2295) for non-carriers (Table 2), indicating that CHEK2 carriers had a significantly higher pCR rate than did non-carriers (P = 0.031; Table 2). In a univariate analysis, other factors associated with improved pCR rates were ER negativity (P < 0.001), PR negativity (P < 0.001), HER2 positivity (P < 0.001), tumor size ≤2 cm (P < 0.001), negative lymph nodes (P < 0.001), and high tumor grade (P < 0.001). The pCR rate was significantly higher in patients who received trastuzumab (43.5%) in combination with neoadjuvant chemotherapy compared with patients who did not (18.6%; P < 0.001) (Table 2).
In the multivariate logistic regression model, CHEK2 H371Y mutation-positive status (odds ratio [OR] = 3.01; 95% confidence interval [CI]:, 1.34 to 6.78; P =0.008), high tumor grade (OR = 2.28; 95% CI: 1.71 to 3.03; P < 0.001), tumor size less than 2 cm (OR = 1.76; 95% CI: 1.38 to 2.24; P = <0.001), negative lymph nodes (OR = 2.10; 95% CI: 1.63 to 2.71; P < 0.001), ER-negativity (OR = 1.99; 95% CI, 1.48 to 2.67; P < 0.001), PR-negativity (OR = 1.65; 95% CI: 1.21 to 2.26; P = 0.002), and concurrent trastuzumab use (OR = 2.42; 95% CI: 1.47 to 3.99; P = 0.001) were independent significant predictors of pCR (Table 3).
In the anthracycline-treated subgroup, CHEK2 mutation carriers had a higher pCR rate than non-carriers (27.8% vs 18.3%), but this was not statistically significant (P = 0.35). In the anthracycline/taxane-treated subgroup, CHEK2 mutation carriers showed a significantly higher pCR rate than non-carriers (50.0% vs 19.7%; P = 0.032). In the taxane-treated subgroup, CHEK2 mutation carriers also had a higher pCR rate than non-carriers (27.3% vs 20.8%), but this was not statistically significant (P = 0.71) (Table 4).
Survival estimates
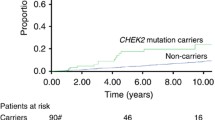
Follow-up data were available for all patients, and the median follow-up time was 38 months (range 1 to 104 months). A total of 258 patients (11.1%) experienced a distant metastases or died of breast cancer during the follow-up period. The estimated 5-year distant recurrence-free survival (DRFS) rate for the entire study population was 84.9% (95% CI: 82.9% to 86.9%). Patients who achieved a pCR had a significantly better 5-year DRFS rate than patients who did not (93.5% vs 82.6%, P < 0.001) (Figure 1A). CHEK2 H371Y mutation carriers had a slightly worse DRFS than did non-carriers (adjusted hazard ratio [HR] =1.24, 95% CI: 0.59-2.63), but the difference did not reach significance (P = 0.57) (Figure 1B). We then stratified the CHEK2 carriers and non-carriers by pCR status. The 5-year DRFS rates for CHEK2 carriers with or without pCR were 100.0% and 81.2%, respectively, whereas the 5-year DRFS rates for non-carriers with or without pCR were 93.6% and 82.7%, respectively (Figure 1C). Patients who achieved a pCR had a better DRFS than who did not in both CHEK2 mutation carriers and non-carriers (P < 0.001). However, CHEK2 carriers without a pCR exhibited the worst DRFS in the four subgroups (Figure 1C).
Kaplan-Meier Estimates of distant recurrence-free survival by pCR andCHEK2H371Y mutation status in 2334 breast cancer patients who received neoadjuvant chemotherapy. Distant recurrence-free survival by pCR status (A); Distant recurrence-free survival by CHEK2 H371Y mutation status (B); Distant recurrence-free survival by pCR and CHEK2 H371Y mutation status (C).
Discussion
In our previous study, we identified a novel recurrent CHEK2 H371Y mutation in Chinese women, and this mutation decreases CHEK2 activity and confers an approximately 2.4-fold increase in breast cancer risk [21]. In the present study, we investigated the association between CHEK2 H371Y and pathologic response in 2334 women who received neoadjuvant chemotherapy. To the best of our knowledge, this is the first study to report that CHEK2 H371Y mutation carriers are more likely to respond to neoadjuvant chemotherapy than are non-carriers and that the H371Y mutation status was an independent favorable predictor of pCR in a multivariate analysis.
In the subgroup analyses, CHEK2 H371Y mutation carriers had a higher pCR rate than did non-carriers in both neoadjuvant anthracycline-based regimens and taxane-based regimens, although the difference did not reach statistical significance. However, the CHEK2 mutation carriers had a significantly higher pCR than did non-carriers among the subgroup of women who received neoadjuvant anthracycline/taxane-containing regimen.
CHEK2 is involved in various DNA damage responses, including cell-cycle checkpoints, genome maintenance, DNA repair and apoptosis [27]. Anthracyclines induce double-strand DNA breaks [28,29], the repair of which is impaired in the deficiency of CHEK2 protein [21,30,31]. Tumor cells that express mutated CHEK2 347 exhibited a 2-to 4-fold increase in apoptosis upon treatment with adrimycin [6]. CHEK2 kinase activity is also required for proper mitotic spindle assembly and chromosome stability [32], and CHEK2 deficient lymphoma cells are more sensitive to taxol [33]. In line with these findings, our results suggested that CHEK2 H371Y was sensitive to both anthracycline and taxane.
Previous studies showed that breast cancer patients with the CHEK2 1100delC mutation had a worse disease-free survival than did patients without this mutation [22,23]. One recent study suggested that breast cancer patients with CHEK2 1100delC mutation had a worse survival beyond 6 years after diagnosis than did non-carriers [34]. In the current study, CHEK2 H371Y carriers showed a slightly poorer DRFS than non-carriers in the entire study cohort with 5-years, it is worth to investigate the survival impact of the CHEK2 H371Y mutation in a long-term follow-up. However, CHEK2 carriers or non-carriers who achieved a pCR had a significant better DRFS than those who did not, whereas CHEK2 H371Y carriers who did not reach a pCR had the worst DRFS in the four subgroups. Although CHEK2 H371Y carriers were more likely to respond to neoadjuvant chemotherapy, only small subset of mutation carriers achieved a pCR, the majority of CHEK2 H371Y carriers who did not reach a pCR might have a particularly aggressive phenotype.
Germline BRCA1 mutation carriers are sensitive to anthracycline or cisplatin neoadjuvant chemotherapy [35-37]; Byrski et al. recently reported that BRCA1 mutation carriers are extremely sensitive to cisplatin-based neoadjuvant chemotherapy (pCR rate 61%, 65 out of 107 patients) [38]. CHEK2 H371Y mutation may share some similarity to BRCA1 mutation, therefore, an interest issue is to see whether CHEK2 H371Y mutation carriers are responsive to cisplatin. Recent clinical trials showed that BRCA1 mutation carriers are sensitive to poly (ADP-ribose) polymerase (PARP) inhibitors [39,40]. In vitro studies suggested that tumor cells with silenced CHEK2 expression showed an increased sensitivity to the PARP 1 inhibitor [33,41]. Therefore, CHEK2 H371Y mutation carriers may be potential candidates for treatment with PARP1 inhibitors.
Although the entire study population is large, the number of individuals with CHEK2 H371Y is relatively small; particularly when the mutation carriers were stratified in several treatment groups, therefore, the results are premature and should be interpreted cautiously.
Nevertheless, our study suggests that patients with a deficiency of CHEK2 activity due to germline mutation, like H371Y, are sensitive to neoadjuvant chemotherapy. It would be of great interest to explore whether other CHEK2 germline mutations (i.e., CHEK2 1100delC) are similarly responsive to neoadjuvant chemotherapy.
Conclusions
Our results suggest that CHEK2 H371Y mutation carriers are more likely to respond to neoadjuvant chemotherapy than are non-carriers. In addition, CHEK2 H371Y mutation may share some similarity to BRCA1 mutation, therefore, CHEK2 H371Y mutation carriers may be potential candidates for treatment with PARP1 inhibitors.
Abbreviations
- A:
-
Anthracycline
- ATM:
-
Ataxia-telangiectasia mutated
- BCS:
-
Breast-conserving surgery
- CHEK2 :
-
Cell-cycle-checkpoint kinase 2
- CI:
-
Confidence interval
- DHPLC:
-
Denaturing high-performance liquid chromatography
- ER:
-
Estrogen receptor
- FISH:
-
fluorescence in situ hybridization
- HER2:
-
Human epidermal growth factor receptor-2
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- OR:
-
Odds ratio
- PARP:
-
Poly(ADP-ribose) polymerase
- pCR:
-
Pathologic complete response
- PgR:
-
Progesterone receptor
- RFS:
-
Recurrence-free survival
- T:
-
Taxane
References
Melchionna R, Chen XB, Blasina A, McGowan CH. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat Cell Biol. 2000;2(10):762–5.
Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282(5395):1893–7.
Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97(19):10389–94.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature. 2001;410(6830):842–7.
Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, et al. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24(2):708–18.
Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287(5459):1824–7.
Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31(1):55–9.
Sodha N, Bullock S, Taylor R, Mitchell G, Guertl-Lackner B, Williams RD, et al. CHEK2 variants in susceptibility to breast cancer and evidence of retention of the wild type allele in tumours. Br J Cancer. 2002;87(12):1445–8.
Vahteristo P, Bartkova J, Eerola H, Syrjakoski K, Ojala S, Kilpivaara O, et al. A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet. 2002;71(2):432–8.
Consortium TCBC. CHEK2*1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74(6):1175–82.
Friedrichsen DM, Malone KE, Doody DR, Daling JR, Ostrander EA. Frequency of CHEK2 mutations in a population based, case–control study of breast cancer in young women. Breast Cancer Res. 2004;6(6):R629–35.
Wu X, Webster SR, Chen J. Characterization of tumor-associated Chk2 mutations. J Biol Chem. 2001;276(4):2971–4.
Bogdanova N, Feshchenko S, Cybulski C, Dork T. CHEK2 mutation and hereditary breast cancer. J Clin Oncol. 2007;25(19):e26.
Weischer M, Bojesen SE, Ellervik C, Tybjaerg-Hansen A, Nordestgaard BG. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26(4):542–8.
Caligo MA, Agata S, Aceto G, Crucianelli R, Manoukian S, Peissel B, et al. The CHEK2 c.1100delC mutation plays an irrelevant role in breast cancer predisposition in Italy. Hum Mutat. 2004;24(1):100–1.
Osorio A, Rodriguez-Lopez R, Diez O, de la Hoya M, Ignacio Martinez J, Vega A, et al. The breast cancer low-penetrance allele 1100delC in the CHEK2 gene is not present in Spanish familial breast cancer population. Int J Cancer. 2004;108(1):54–6.
Shaag A, Walsh T, Renbaum P, Kirchhoff T, Nafa K, Shiovitz S, et al. Functional and genomic approaches reveal an ancient CHEK2 allele associated with breast cancer in the Ashkenazi Jewish population. Hum Mol Genet. 2005;14(4):555–63.
Kleibl Z, Novotny J, Bezdickova D, Malik R, Kleiblova P, Foretova L, et al. The CHEK2 c.1100delC germline mutation rarely contributes to breast cancer development in the Czech Republic. Breast Cancer Res Treat. 2005;90(2):165–7.
Adank MA, Jonker MA, Kluijt I, van Mil SE, Oldenburg RA, Mooi WJ, et al. CHEK2*1100delC homozygosity is associated with a high breast cancer risk in women. J Med Genet. 2011;48(12):860–3.
Liu Y, Liao J, Xu Y, Chen W, Liu D, Ouyang T, et al. A recurrent CHEK2 p.H371Y mutation is associated with breast cancer risk in Chinese women. Hum Mutat. 2011;32(9):1000–3.
de Bock GH, Schutte M, Krol-Warmerdam EM, Seynaeve C, Blom J, Brekelmans CT, et al. Tumour characteristics and prognosis of breast cancer patients carrying the germline CHEK2*1100delC variant. J Med Genet. 2004;41(10):731–5.
Schmidt MK, Tollenaar RA, de Kemp SR, Broeks A, Cornelisse CJ, Smit VT, et al. Breast cancer survival and tumor characteristics in premenopausal women carrying the CHEK2*1100delC germline mutation. J Clin Oncol. 2007;25(1):64–9.
Yao L, Liu Y, Li Z, Ouyang T, Li J, Wang T, et al. HER2 and response to anthracycline-based neoadjuvant chemotherapy in breast cancer. Ann Oncol. 2011;22(6):1326–31.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85.
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–9.
Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4(3):216–25.
Di Leo A, Chan S, Paesmans M, Friedrichs K, Pinter T, Cocquyt V, et al. HER-2/neu as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Breast Cancer Res Treat. 2004;86(3):197–206.
Lal S, Mahajan A, Chen WN, Chowbay B. Pharmacogenetics of target genes across doxorubicin disposition pathway: a review. Curr Drug Metab. 2010;11(1):115–28.
Darbon JM, Penary M, Escalas N, Casagrande F, Goubin-Gramatica F, Baudouin C, et al. Distinct Chk2 activation pathways are triggered by genistein and DNA-damaging agents in human melanoma cells. J Biol Chem. 2000;275(20):15363–9.
Theard D, Coisy M, Ducommun B, Concannon P, Darbon JM. Etoposide and adriamycin but not genistein can activate the checkpoint kinase Chk2 independently of ATM/ATR. Biochem Biophys Res Commun. 2001;289(5):1199–204.
Stolz A, Ertych N, Kienitz A, Vogel C, Schneider V, Fritz B, et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol. 2010;12(5):492–9.
Hoglund A, Stromvall K, Li Y, Forshell LP, Nilsson JA. Chk2 deficiency in Myc overexpressing lymphoma cells elicits a synergistic lethal response in combination with PARP inhibition. Cell Cycle. 2011;10(20):3598–607.
Kriege M, Hollestelle A, Jager A, Huijts PE, Berns EM, Sieuwerts AM, et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: impact of adjuvant chemotherapy. Br J Cancer. 2014;111(5):1004–13.
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–9.
Byrski T, Huzarski T, Dent R, Gronwald J, Zuziak D, Cybulski C, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2009;115(2):359–63.
Chappuis PO, Goffin J, Wong N, Perret C, Ghadirian P, Tonin PN, et al. A significant response to neoadjuvant chemotherapy in BRCA1/2 related breast cancer. J Med Genet. 2002;39(8):608–10.
Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–5.
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly (ADP-ribose) polymerase. Nature. 2005;434(7035):913–7.
McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109–15.
Acknowledgements
This study was supported by the 973 project 2013CB911004; program for Breast Cancer Tissue Bank of Beijing, and grants from the National Natural Science Foundation of China (No. 30973436, No. 81071629 and No. 81202107).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: YX. Performed the experiments: YL YX. Analyzed the data: YL YX. Contributed reagents/materials/analysis tools: TO JL TW ZF TF BL. Wrote the paper: YX YL. All authors read and approved the final manuscript.
Yin Liu and Ye Xu contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Liu, Y., Xu, Y., Ouyang, T. et al. Association between CHEK2 H371Y mutation and response to neoadjuvant chemotherapy in women with breast cancer. BMC Cancer 15, 194 (2015). https://doi.org/10.1186/s12885-015-1203-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1203-3