Abstract
Background
Frailty can be defined as a progressive loss of reserve and adaptive capacity associated with an overall deterioration in health that can result in disability, loss of independence, hospitalisation, extensive use of healthcare resources, admission to long-term care and death. Nevertheless, despite widespread use of the term, there is no agreement on the definition of frailty or an instrument to identify it in a straightforward way. The purpose of the current study was to explore which factors are associated with frailty-related adverse outcomes in elderly individuals and to propose a suitable tool for identifying such individuals, particularly in primary care settings.
Methods
A prospective open cohort study of community dwelling, independent individuals aged 75 or over, followed up for 2 years. The study was entirely conducted in a primary care setting. Study variables included independence status measured by Barthel’s Index and the Lawton Instrumental Activities of Daily Living Scale, functional performance, assessed by Timed Up and Go (TUG) and Gait Speed (GS) tests and levels of polipharmacy, comorbidity and social support. Outcome variables were specific frailty-related adverse events, namely, loss of independence and death.
Results
Overall, 215 community-dwelling independent individuals initiated the study. Of these, 46 were lost to follow-up and 50 had frailty-related adverse events during the follow-up period. Individuals with adverse events during the study had poorer functional status at baseline. The multivariate model that best explained the occurrence of these events included the variables of age, presence of polipharmacy and the TUG time. The AUC (Area under the curve) of this model was 0.822.
Conclusions
Given the simplicity of assessing the three derived factors and their combined discriminant power, the proposed model may be considered a suitable tool for identifying frail patients, i.e., people more likely to lose their independence or die within a relatively short time interval.
Similar content being viewed by others
Background
Frailty can be defined as a progressive loss of reserve and adaptive capacity associated with an overall deterioration in health that can result in disability, loss of independence, hospitalisation, extensive use of healthcare resources, admission to long-term care and death [1–3]. Nevertheless, despite the widespread use of the term, there is no agreement on the definition of frailty [4, 5] or on an instrument to identify it [6] in a straightforward way, particularly in primary care.
Three approaches for the identification of frail individuals have been described in the literature [7]: the rules-based, the cumulative and the clinical judgment. Rules-based approaches are derived from multiple regression models and are based on the presence of a number of symptoms. The phenotypic approach would be included in this group [1]. Cumulative-based approaches are based on the consideration and addition of the number of impairments presented by a single person [8]. Finally, clinical judgment based approaches rely on the professional interpretation of the clinical record and physical examinations [7].
A frequently considered aspect in the aforementioned approaches is functional performance. Several instruments have been proposed to assess various aspects of functional performance such as motor strength, mobility and balance. Those most commonly used being the Timed Up and Go (TUG) [9, 10] and Gait Speed (GS) tests [11, 12]. Such tests have demonstrated their usefulness in terms of monitoring the health status, functional capacity and risk of falls of elderly people [11, 13–15].
Early identification of frail individuals in primary care could have a double impact. On the one hand, it may help improve the health status of identified individuals, since it would be possible to address their needs in a comprehensive manner, delaying or preventing the natural progression towards dependence [16]. On the other hand, it may lead to reductions in resources use [2].
The objective of this study was to assess which factors are associated with frailty-related adverse outcomes in elderly individuals and on the basis of these results and from a rules-based perspective, to propose a suitable instrument for identifying these patients in primary care.
Methods
Study population and recruitment
An open cohort of community-dwelling individuals, aged 75 years or more and independent (Barthel’s index ≥90) at the time of inclusion. Patients of advanced age were selected in order to assure a rate of occurrence of the defined adverse outcomes, adequate to the project design and span [17, 18]. Recruitment took place in the primary care centres of two municipalities in Gipuzkoa (Spain) and subjects were followed up for 2 years. The participating health centres are situated in an urban area close to the provincial capital (San Sebastian) and their population characteristics are similar to the Basque Country region in terms of age, sex and deprivation index [19]. These centres provide primary care services to a total adult population of around 30,000 individuals. The study was carried out between July 2010 and December 2013.
Eligible individuals were selected randomly from administrative databases in order to obtain a representative sample of the reference population in terms of age and sex. After being informed about the study by their corresponding primary care doctors they were invited to a meeting where detailed information about the research project was presented and informed consent was provided. The study was approved by the Clinical research ethics committee of the Gipuzkoa health region (ref.: 05/2010).
Individuals were excluded from the study if they were institutionalised, planned to move within the following 2 years or were included in a home care programme for chronic health problems. Patients with impaired cognitive function, defined as 5 or more errors on the Pfeiffer’s Short Portable Mental Status Questionnaire [20, 21], and those who were dependant, defined as a score <90 in the Barthel’s index [22] were also excluded. All participants provided informed consent.
Study variables
As main outcome variables were considered the events of death and loss of independence; the latter defined as loss of ≥ of 10 % of the baseline Barthel score during the study follow-up period, considering that this reduction on Barthel score imply the loss of independence in preforming a basic daily living activity [23–27].
Interviews were performed on an individual basis and information collected at baseline included among others: age, sex, level of education, living arrangements, and social risk measured using the Gijon Scale [28]. To assess participants’ functional status, the Lawton Instrumental Activities of Daily Living (IADL) Scale [23, 29] and two physical functional performance tests (TUG and GS) [9, 11] were used. To assess overall health status, information was requested regarding loss in body weight, level of physical exercise, sensory deficits including hearing impairment, and self-perceived health using the following question: “How would you rate your health?”
Additionally, health records were reviewed to detect any falls and hospital admissions in the previous year; to confirm the presence/absence of polipharmacy, defined as the simultaneous prescription of 4 or more drugs continuously for 3 or more months [30]; and/or comorbidity, defined as three or more of the following conditions: stroke with sequelae, myocardial infarction or recently diagnosed heart failure, Parkinson’s disease, chronic obstructive pulmonary disease, musculoskeletal disorders resulting in pain or functional impairment, diabetes mellitus treated orally or with insulin, neurological gait disorders, unresolved urinary complaints, and mental illness under treatment with psychoactive medication [31]. The interviews and review of health records were carried out by two nurses trained for the purpose.
Follow-up
Every six months from the date of recruitment and over the 2 years of follow-up, patients were appointed for an additional assessment. These assessments included all the aforementioned functional and daily activity measures (Barthel’s index, Lawton’s scale, TUG and GS tests) and information about weight, physical exercise, sensory deficits and self-perceived health. The corresponding nurse was contacting patients not attending a follow-up assessment visit. Those stating that they lost interest in the study were considered drop-outs. Patients who lost independence and had to be admitted to an older people’s home were registered as dependent. If the family took care of the dependent individuals, Barthel values were also collected by interviewing the person responsible to provide for them. Death events were verified by medical records. Patients who could not be located and did not come back for the rest of the assessments were considered drop-outs. Patients, who returned after missing certain assessments, were kept in the final sample and were assigned in the corresponding outcome group. No missing values imputation was performed for the needs of this study.
Sample size
Loss of independence and mortality rates in subjects over 75-years of age were reviewed in previous studies in our setting (non-published data). Obtained estimates were equal to 18 and 5 % respectively. Assuming a dropout rate of 10 % during the follow-up period, we calculated that with n = 200 participants we could expect around 40 individuals to lose their independence by the end of the study. Given the binary nature of the considered outcome (deterioration Yes/No), applying the rule of thumb of 10 events per variable this sample size would enable us to construct binary logistic regression models including simultaneously 4 to 5 predictive factors, should results indicate that many factors to be relevant.
Statistical analysis
The unit of analysis was the patient. Descriptive statistics were used to analyse dependent and independent variables at baseline and each follow-up assessment. Frequencies and percentages were calculated for categorical data and means and standard deviations (SD) or medians and interquartile ranges (Q1, Q3) for continuous data, depending on their distribution. Categorical variables were compared with chi-squared or Fisher’s exact tests while Student’s t-test or the non-parametric Wilcoxon were implemented for normally and non-normally distributed continuous variables.
In order to assess whether missing data were random or were associated with specific patient characteristics, socio-demographic and clinical characteristics variables were compared between lost to follow-up individuals and those who completed the follow-up. Finally, univariate and multivariate binary logistic regression models were constructed to assess the relationship between each of the independent variables and the occurrence of the frailty-related adverse events under study. All variables with significance levels p ≤ 0.10 in the univariate analysis were considered for the construction of the multivariate model. At this stage both backward and forward stepwise models were tested. In the backward procedure all relevant variables entered in the same model. Non-significant variables were eliminated one-by-one, based on their p-values (eliminating the less significant first), until no p-value >0.05 were left in the model. In the forward procedure, the most significant variable entered in the model, followed by the next more significant etc., eliminating those with p-value > 0.05 [32].
In a secondary stage sensitivity analyses were performed. Further multivariate models were fitted, making different assumptions about the drop-out patients. In particular it was assumed that: a) all lost to follow-up had a negative outcome; b) all lost to follow up had a positive outcome; c) those not located had a negative outcome and finally d) those not located had a positive outcome.
The diagnostic performance and goodness-of-fit statistics were studied for all constructed models. The results of the final model are presented in terms of odds ratios (OR) with corresponding 95 % confidence intervals (CI). The area under the curve (AUC), Hosmer-Lemeshow statistic and R-square values are also reported. Results were considered statistically significant when p <0.05. All analyses were performed with the SAS 9.3 software.
Results
A total of n = 215 individuals were initially included in the study and of those n = 169 completed the proposed follow-up. No significant differences were found in terms of socio-demographic and clinical characteristics between lost to follow-up subjects and those who completed the study. At the end of the follow-up period, n = 119 subjects remained independent, while the other n = 50 participants (24 %) had a frailty-related adverse outcome: death (n = 8) or loss of independence (n = 42) (Fig. 1). The rates of independence loss in the first and second years were 8.3 and 2.6 %, respectively.
At baseline, participants had a mean age of 79.4 (SD: 4.1) years and 63 % were women. A high proportion of subjects presented comorbidity (72 %) and polipharmacy (61 %). Comparing the baseline status of those who eventually developed a frailty-related adverse outcome and those who did not, we observed the following. The adverse outcome group was on average 3 years older (p < 0.001), had a lower level of physical activity (p = 0.001) and was more likely to present polipharmacy (p = 0.001) or comorbidity (p = 0.032). In addition, it presented more hospital admissions in the previous year (p = 0.013) and declared a poorer self-perceived health status. No significant differences were found in the variables of sex, body mass index, visual or auditory deficits and accidental falls in the previous year (Table 1).
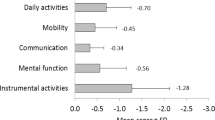
As far as functional status is concerned, participants who developed frailty-related adverse outcomes, despite being independent at baseline, had lower levels of functioning in terms of basic activities of daily living (Barthel’s index) and IADL (Lawton’s score). Significant differences were also observed in the functional performance tests of TUG and GS at baseline. Participants with frailty-related adverse outcomes performed worse in both (p < 0.001) (Table 2). No socio-demographic differences were observed between those finishing the study and drop-out subjects. The latter were half a year older than the rest (mean drop-out age 79.9 (SD: 4.0), p = 0.398); 39 % of them were men, compared to 36 % of those completing the study (p = 0.705). Both groups had similar baseline Barthel values (p = 0.901) and self-perceived health (p = 0.218).
Except for Barthel and Lawton scales, all other variables with p-values ≤0.10 presented in Tables 1 and 2 were implemented in the construction of the multivariate logistic regression model. It’s worth mentioning that due to their high correlation (r = −0.821), GS and TUG could not be both considered in the same model and the TUG was selected given its capacities [33]. Furthermore, the presence of comorbidity and having specific health problems (e.g., musculoskeletal disorders) were not significant in any of the models that included the polipharmacy variable, possibly due to the strong association between these factors. Both the forward and backward stepwise regressions resulted in the same multivariate model.
The multivariate logistic regression model that best fitted the data included age (OR: 1.14; 95 % CI: 1.03, 1.25), polipharmacy status (OR: 2.74; 95 % CI: 1.06, 7.06) and TUG time (in seconds) (OR: 1.28, 95 % CI: 1.14, 1.44) (Table 3). Our results suggest that these factors have good discriminant validity, with an area under the curve (AUC) of 0.822 (Fig. 2), adjusted R-squared of 0.384 and Hosmer Lemeshow p = 0.721 (Table 3).
ROC of the proposed model for identifying frailty in primary care. Receiver operating characteristic curve for the final model to predict frailty-related outcomes, based on age, Timed Up and Go time and polypharmacy status. The curve represents the relationship between sensitivity and 1-specificity for all potential cut-off points of the diagnostic test under study. The area under the curve (AUC), a measure of the discriminatory power of the test, is 0.822. The cut-off point that maximises sensitivity and specificity (i.e., 76 %) is 0.288
The additional sensitivity analyses gave the following results. When all lost to follow-up (n = 46) were assumed to be finally dependent, the derived model included the variables of age, TUG and at least one hospital admission in the last year. When only those not located (n = 29) were considered dependent, the model included age and TUG. When all lost to follow-up (n = 46) or only those not located (n = 29) were assumed to be independent, the final models included the variables of age, TUG and polipharmacy. The AUCs of all four models oscillated between 0.733 and 0.795.
Discussion
Proper identification of frail individuals in primary care represents an unresolved challenge. The current study, leaving aside discussions on the definition of frailty and its components, addresses this issue from a pragmatic point of view. Subjects able to perform basic daily living activities (BADL), so independent, are considered frail when they lose independence or die in a maximum period of two years. With this approach, the prevalence of frailty in our sample would be 24 %, higher than expected in our setting, but in agreement with other published studies [4, 34].
Our study shows that it is possible to identify clear differences between groups of community-dwelling elderly individuals that do and do not lose their independence within 2 years. The differences are significant in terms of age, level of physical activity, health status and functional capacity. The current results are in agreement with the literature on this topic, indicating association between frailty with age, comorbidity, self-perceived health and functional status [35]. No differences in terms of socioeconomic status, or family and social network were found in our sample.
Considering the prospective nature of the methodology used in this study and the rules-based approach proposed for the construction of operational definitions of frailty, we found that the factors associated with frailty-related adverse outcomes could be expressed in a single model including age, polipharmacy status and TUG time. We should underline that when constructing the model polipharmacy was selected over other comorbidity-related variables, as it is easier to assess than other comorbidity indices. As stated in the results section, none of these other variables was statistically significant when considered simultaneously with polipharmacy, most likely due to the existing relations between them. The sensitivity analyses performed seems to support the presented model. Even though hospital admission turned out to be significant, when all drop-outs were considered dependent, it is a fact that hospital admission and polipharmacy are not independent, rather highly related variables. Recent hospital admission may be indicative of a health problem which can end up in a negative outcome and has been often described as associated to frailty [36]. A high association between the two was also found in our in our sample (p = 0.002).
To date, a variety of approaches have been suggested for identifying frail individuals. One well-known approach uses the frailty phenotype proposed by Fried in 2001 [1], based on the presence of at least three of the following deficits: slow gait speed, weak grip strength, low level of physical activity, exhaustion and unintentional weight loss. Despite the widespread use of this phenotype in research, this tool has not been widely adopted in clinical practice, possibly due to the lack of population studies establishing diagnostic cut-offs for some of the criteria [3]. Other ways of identifying frail individuals involve frailty indices, based on cumulative approaches [37]. Indices published in recent years include the Survey of Health, Ageing and Retirement in Europe (SHARE) Frailty Instrument [38, 39], and the Program of Research to Integrate Services for the Maintenance of Autonomy (PRISMA) questionnaire [40]. These indices assess the existence of deficits in areas such as strength, balance, nutrition, resistance, mobility, physical activity and cognitive ability, but none of them have been taken up in primary care settings. Also, a third approach based on the clinical judgment it’s being explored. In this group, the Gérontopôle Frailty Screening Tool [41] and the EASY-Care TOS [42, 43] can be considered. The latter, with a specific focus on primary care settings, provides a robust and easy to perform two step tool for the identification of frail patients in primary care practices. It is interesting to note the parallelisms with this article especially regarding aim (primary care oriented) and methodology (longitudinal, adverse outcome based). Nevertheless, the main difference is the implemented frailty identification approach. Our tool is a rules-based one while EASY-Care TOs is based on clinical judgment. Additionally, our proposal overcomes one of the main limitations of the clinical judgment based tools, which is that they rely on a strong and long term patient –care professional relationship, whereas the proposed tool is based on three objective and easy to measure variables, not requiring previous knowledge of the patient’s clinical history. Hence, given the characteristics and simplicity of our model, we believe that once validated in a different and broader population, it could be considered as a screening test for identifying frail individuals in primary care.
The main limitation of our study is related to its cohort design and associated losses to follow-up. To minimise this bias, we thoroughly informed patients about the goals of the research, arranged informative meetings with them and carried out a campaign to raise awareness of the study in the participating health centres. There were more losses during the follow-up period than expected, but figures were similar to those of other studies and are considered acceptable [44]; further, we reached the number of frailty-related adverse outcomes expected, which has enabled us to achieve the desired robustness in the analysis. Another notable limitation is the fact that repeating performance tests may lead to a certain degree of training. In any case, this limitation would underestimate the onset of functional deterioration and hence would not affect the validity of the results [45]. To minimise bias from inter-observer differences, we standardised the criteria for the administration of the various different scales and tests and trained the nurses in charge of data collection.
The main strength of this study is the fact that it was conducted entirely in a primary care setting. Many studies in this field are conducted in geriatric settings, where population characteristics and patient needs are very different. A recently initiated research project (reference PI14/01905) will allow us to further validate the ability of the proposed model in identifying frail individuals in a larger sample of independent community dwelling elders aged 70 or more.
Conclusions
It is possible to identify frail individuals considering their age, polipharmacy status and functional capacity. These three factors can be assessed in a simple and quick way, fact which renders the proposed model suitable to use in primary care.
Ethics approval and consent to participate
All participants received complete information about the project and written informed consent was provided. The study was approved by the Clinical Research Ethics Committee of the Gipuzkoa Health Region (ref.: 05/2010).
Consent for publication
Not applicable.
Availability of data and materials
The database set was available for all authors of the study and will be available for other non-commercial researches on request.
Abbreviations
- AUC:
-
area under the curve
- BADL:
-
basic activities of daily living
- CI:
-
confidence intervals
- GS:
-
gait speed
- IADL:
-
Instrumental activities of daily living
- OR:
-
odds
- PRISMA:
-
Program of research to integrate services for the maintenance of autonomy
- SD:
-
standard deviations
- SHARE:
-
Survey of health, ageing and retirement in Europe
- TUG:
-
Timed up and go
References
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56 (3).
Lacas A, Rockwood K. Frailty in primary care: a review of its conceptualization and implications for practice. BMC Med. 2012;10:4.
Garcia-Garcia FJ, Alfaro AA. Fragilidad: de la epidemiología a la clínica. Rev Esp Geriatr Gerontol. 2010;45:250–1.
Santos-Eggimann B, Karmaniola A, Seematter-Bagnoud L, Spagnoli J, Bula C, Cornuz J, et al. The Lausanne cohort Lc65+: a population-based prospective study of the manifestations, determinants and outcomes of frailty. BMC Geriatr. 2008;8:20.
Pialoux T, Goyard J, Lesourd B. Screening tools for frailty in primary health care: a systematic review. Geriatr Gerontol Int. 2012;12:189–97.
Van Abellan KG, Rolland Y, Houles M, Gillette-Guyonnet S, Soto M, Vellas B. The assessment of frailty in older adults. Clin Geriatr Med. 2010;26:275–86.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95.
Rockwood K, Mitnitski AB, MacKnight C. Some mathematical models of frailty and their clinical implications. Rev Clin Gerontol. 2002;12:109–17.
Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. 1986;67:387–9.
Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8.
Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60:1304–9.
Varela PL, Ortiz Saavedra PJ, Chavez JH. Velocidad como indicador de fragilidad en adultos mayores de la comunidad en Lima, Perú. Rev Esp Geriatr Gerontol. 2010;45:22–5.
Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people--results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–80.
Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. 2010;58:844–52.
De LJ, Degryse J, Illiffe S, Mann E, Buntinx F. Family physicians need easy instruments for frailty. Age Ageing. 2008;37:484–5.
Drubbel I, Numans ME, Kranenburg G, Bleijenberg N, de Wit NJ, Schuurmans MJ. Screening for frailty in primary care: a systematic review of the psychometric properties of the frailty index in community-dwelling older people. BMC Geriatr. 2014;14:27.
Gobbens RJ, van Assen MA. Frailty and its prediction of disability and health care utilization: the added value of interviews and physical measures following a self-report questionnaire. Arch Gerontol Geriatr. 2012;55:369–79.
Jurschik P, Nunin C, Botigue T, Escobar MA, Lavedan A, Viladrosa M. Prevalence of frailty and factors associated with frailty in the elderly population of Lleida, Spain: the FRALLE survey. Arch Gerontol Geriatr. 2012;55:625–31.
Osakidetza. Osakidetza. 1-10-2015. Ref Type: Electronic Citation. http://www.osakidetza.euskadi.eus/r85-phosag01/es. Accesed 10 Jan 2015.
Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41.
Martínez Dela Iglesia J, Dueñas Herrero R, Onís Vilches MC, Aguado Taberné C, Albert Colomer C, Luque Luque R. Adaptación y validación al castellano del cuestionario de Pfeiffer (SPMSQ) para detectar la existencia de deterioro cognitivo en personas mayores de 65 años. Med Clin (Barc). 2001;117:129–34.
Ward G, Jagger C, Harper W. A review of instrumental ADL assessments for use with elderly people. Rev Clin Gerontol. 1998;8:65–71.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–9.
Cid-Ruzafa J, Damian-Moreno J. [Disability evaluation: Barthel’s index]. Rev Esp Salud Publica. 1997;71:127–37.
Vergara I, Vrotsou K, Orive M, Gonzalez N, Garcia S, Quintana JM. Factors related to functional prognosis in elderly patients after accidental hip fractures: a prospective cohort study. BMC Geriatr. 2014;14:124.
Vergara I, Vrotsou K, Orive M, Garcia-Gutierrez S, Gonzalez N, Las HC, et al. Wrist fractures and their impact in daily living functionality on elderly people: a prospective cohort study. BMC Geriatr. 2016;16:11.
Alarcón AT, nzalez Montalvo JI. La escala socio-familiar de gijón, instrumento útil en el hospital general. Rev Esp Geriatr Gerontol. 1998;33:178–9.
Vergara I, Bilbao A, Orive M, Garcia-Gutierrez S, Navarro G, Quintana JM. Validation of the spanish version of the Lawton IADL scale for its application in elderly people. Health Qual Life Outcomes. 2012;10:130.
Martin LI, Lopez-Torres Hidalgo JD, Gorronogoitia IA, De-Hoyos Alonso MC, Baena Diez JM, Herreros HY. [Preventive activities in the elderly]. Aten Primaria. 2014;46 Suppl 4:75–81.
Badia X. Estudios sobre la calidad de vida de personas afectas de diferentes patologías. Edited by Ministerio de Sanidad y política social. 2009. Madrid. Ref Type: Report.
Steyerberg EW. Clinical prediction models. Springer; 2009. ISBN 978-0-387-77243-1 DOI 10.1007/978-0-387-77244-8.
Herman T, Giladi N, Hausdorff JM. Properties of the ‘timed up and go’ test: more than meets the eye. Gerontology. 2011;57:203–10.
Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64:675–81.
Sousa AC, Dias RC, Maciel AC, Guerra RO. Frailty syndrome and associated factors in community-dwelling elderly in Northeast Brazil. Arch Gerontol Geriatr. 2012;54:e95–e101.
Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–31.
Rockwood K, Hogan DB, MacKnight C. Conceptualisation and measurement of frailty in elderly people. Drugs Aging. 2000;17:295–302.
Romero-Ortuno R. The frailty instrument for primary care of the survey of health, ageing and retirement in Europe predicts mortality similarly to a frailty index based on comprehensive geriatric assessment. Geriatr Gerontol Int. 2013;13:497–504.
Romero-Ortuno R, O’Shea D, Kenny RA. The SHARE frailty instrument for primary care predicts incident disability in a European population-based sample. Qual Prim Care. 2011;19:301–9.
Applegate WB, Miller ST, Graney MJ, Elam JT, Burns R, Akins DE. A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital. N Engl J Med. 1990;322:1572–8.
Vellas B, Balardy L, Gillette-Guyonnet S, Van Abellan KG, Ghisolfi-Marque A, Subra J, et al. Looking for frailty in community-dwelling older persons: the gerontopole frailty screening tool (GFST). J Nutr Health Aging. 2013;17:629–31.
van Kempen JA, Schers HJ, Melis RJ, Olde Rikkert MG. Construct validity and reliability of a two-step tool for the identification of frail older people in primary care. J Clin Epidemiol. 2014;67:176–83.
van Kempen JA, Schers HJ, Philp I, Olde Rikkert MG, Melis RJ. Predictive validity of a two-step tool to map frailty in primary care. BMC Med. 2015;13:287.
Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93:458–61.
The Epidemiology of Aging. Springer; 2012. ISBN 978-94-007-5060-9 DOI 10.1007/978-94-007-5061-6.
Acknowledgements
Authors wish to thank all the participants who took part on this study for their interest and generous dedication.
Funding
Gobierno Vasco- Eusko Jaurlaritza. Departamento de Sanidad y Consumo. Ayudas investigación sanitarias 2011. Proyecto N°: 2011111122.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AD, AB, JN, KV, IS, IV have made substantial contributions to conception and design of the study; KV, performed the analysis and IV, AB, AD, IS interpreted the data; IV, KV have been involved in drafting the manuscript and AD revised it critically for important intellectual content; AD, AB, JN, KV, IS, IV have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Diez-Ruiz, A., Bueno-Errandonea, A., Nuñez-Barrio, J. et al. Factors associated with frailty in primary care: a prospective cohort study. BMC Geriatr 16, 91 (2016). https://doi.org/10.1186/s12877-016-0263-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-016-0263-9