Abstract
This paper presents the structural, optical and photoluminescence properties of wet chemically synthesized ZnO:Pd2+ colloidal nanocrystals characterised by X-ray diffraction, scanning electron microscopy/energy-dispersive X-ray spectroscopy (EDS) and Fourier transform infrared spectroscopic techniques. Increase in lattice parameters from diffraction data indicates the incorporation of Pd2+ in the ZnO crystal lattice. A small amount of dopant favours the formation of stoichiometric ZnO nanoparticles; otherwise, non-stoichiometric nanocrystal formation was observed from the EDS data. The optical gap was found to decrease with the doping concentration, except for the small dopant level of 0.05% of Pd2+ where an increase in the optical gap was observed. Intensities of characteristic luminescence bands for pure ZnO nanocrystals (357, 387 and 420 nm) were found to decrease with the increasing Pd2+ concentration, and two new bands centred at 528 and 581 nm for 0.5% Pd2+ concentration were observed. These results have been explained on the basis of change in the oxygen vacancy-related defects and/or formation of new trap states which in turn affect the luminescence properties of ZnO:Pd2+ nanocrystals, which are important in the realisation of visible light-emitting solid-state devices.
Similar content being viewed by others
Background
Transition metal-doped metal oxide nanocrystals have attracted considerable interest in the scientific community due to their unique optical and electro-optical properties. Among them, zinc oxide (ZnO) which has a wide bandgap (3.37 eV) and high exciton binding energy (60 meV) is a potential host material for doping transition metal ions [1]. Various theoretical and experimental studies on ZnO reveal a much wider scope in terms of nanocrystal shapes (wire, rod, cone and spherical), lattice structures, doping, surface modifications as well as synthesis conditions for tailoring the physical and optical properties of these nanocrystals [2–5]. Various dopants such as transition metals, Mn, Fe, Co and Ni, and rare earth elements, Eu, Er and Tb, have successfully been incorporated into the colloidal semiconducting nanocrystals (ZnO) for tailoring their characteristic properties [6]. The resulting material combinations have novel magnetic properties, enhanced optical absorption, better luminescence effects and improved sensing response, which make them suitable for gas sensor device applications [7]. Pd2+-doped ZnO shows good stability to a wide range of chemical compounds along with enhancement in sensitivity and response time [8–11]. CO gas sensing characteristics, such as sensitivity to low concentrations, selectivity and response and recovery times, were found to improve in Pd-doped ZnO nanofibers synthesized by electrospinning method [11]. Recently, it was reported that decorating ZnO nanorod arrays with Pd nanoparticles enhances the gas sensing characteristics due to the acceleration in sensing reaction [12]. The enhancement in gas sensing activity with the addition of Pd (5 at %) for ZnO nanostructures has also been reported by Gondal et al. [13]. The significance of Pd-doped nanostructures can be understood properly by knowing the effect of composition on various structure/property relationships. Therefore, in view of the importance of Pd doping, we are reporting the structural, optical and fluorescent properties of colloidal ZnO:Pd2+ nanocrystals synthesized by wet chemical precipitation technique.
Methods
Pd-doped ZnO nanocrystals were grown by wet chemical method based on alkaline-activated hydrolysis and condensation of zinc acetate in dimethyl sulfoxide (DMSO) to form ZnO nanocrystals [7]. Analytical grade reagents without any further purification were used in this study. A 2.5-mmol solution of tetramethyl ammonium hydroxide mixed in dry ethanol (5 ml) was added dropwise to a solution of 0.1 M zinc acetate solution in DMSO (15 ml) under vigorous stirring at room temperature. The same method was also used for synthesizing the Pd-doped ZnO by varying the palladium acetate concentration (0.05, 0.1, 0.2 and 0.5 mol%) during the reaction. Synthesis with higher dopant concentrations resulted in a black residue with very little yield. Suspended nanocrystals were precipitated from DMSO by adding a non-solvent such as ethyl acetate (40 ml), and the nanocrystals were re-suspended in dry ethanol. The process was repeated three times, and the aggregates of pure ZnO and ZnO:Pd2+ nanocrystals were air-dried to get the final powders. For surface passivation, the known quantity of powders was added to the molten trioctylphosphine oxide (TOPO), stirred at 150°C for 1.5 h, then cooled, washed with ethanol and finally centrifuged repeatedly to remove the excess TOPO. The stabilised nanoparticles were finally suspended in toluene to form stable, high-optical-quality colloidal solutions for optical absorption and fluorescence studies.
The synthesized nanocrystals were characterised by X-ray diffraction (XRD) (D8 Focus, BRUKER, Ettlingen, Germany) operated at 40 kV and 20 mA using Cu-Kα1 radiation. Surface morphology and dopant concentrations were obtained by scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDS) (JEOL 6610 LV, JEOL Ltd., Akishima, Tokyo, Japan). Fourier transform infrared spectroscopy (FTIR) was used to study the molecular structure in the (4,000 to 400 cm−1) spectral region using the KBr pallet method (660 Varian Resolution, Palo Alto, CA, USA). Absorption spectra of the colloidal solutions in quartz cuvettes with an optical path length of 1 cm were collected using a UV–vis spectrophotometer (Lambda 35, PerkinElmerInstruments, Branford, CT, USA). Photoluminescence emission spectra at 350 nm of excitation energy were recorded using a fluorescence spectrometer (Lambda 45, PerkinElmer Instruments).
Results and discussion
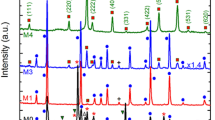
Figure 1 shows the XRD patterns for pure and Pd2+-doped ZnO nanocrystals. The observed strong diffraction peaks (100), (002), (101), (102), (110), (103), (201), (112) and (200) for all samples are consistent with the wurtzite ZnO phase (JPCDS 36–1451). The crystallite size, determined from X-ray line broadening using Debye Scherrer's formula, was found to be 10.5 ± 1.5 nm for all samples. The broadening of the diffraction peaks indicates different crystal sizes which form different polycrystalline aggregates [14, 15]. The crystallite size and strain depend on the 2θ peak position which enables us to determine the effect of peak broadening using the Williamson-Hall (W-H) method [16, 17]:
The plot of 4 sinθ versus β cosθ taking (100), (002), (101), (102), (110) and (103) lattice planes corresponding to the wurtzite phase of ZnO are shown in Figure 2. From the linear fit to the data, the crystallite size D was extracted from the y-intercept and the strain ε from the slope of the straight line. The strain is due to the incorporation of a dopant in the periodic lattice. The W-H plots show a negative strain for pure and Pd2+-doped ZnO nanoparticles which is an indication of lattice shrinkage [17].
The effect of this strain can be evaluated by calculating the lattice parameters such as the interplanar spacing d, the lattice constants (a, b and c) and the unit cell volume V which were calculated from the lattice geometry equations as given below [17]:
The obtained lattice parameters along with particle size for different samples are summarised in Table 1. Similar values of the lattice parameters have been reported for the undoped ZnO nanoparticles [17]. It has been observed that the lattice parameters (a and c) increases with the increase in dopant concentration. Pd ions substitute for the isovalent Zn ions at the octahedral interstitial site as compared to the tetrahedral site without significant lattice distortion in the ZnO crystallites [18]. The variation in lattice dimension with Pd2+ content preliminarily implies that Pd2+ was substituted for Zn2+ in ZnO; the detailed effect on optical properties will be discussed later in this paper.
The SEM images and EDS spectra for the as-synthesized nanocrystals are shown in Figure 3, and the relative atomic compositions for each sample are summarised in Table 2. The R values, i.e. the ratio of number of anions to cations (O2−/(Zn, Pd)2+) for a given sample, are also tabulated. It is observed that for small dopant concentrations (0.05% Pd2+), the samples were near stoichiometric.
Figure 4 shows the FTIR spectra of pure and doped ZnO:Pd2+ nanocrystals. The peak at 3,370 ± 20 cm−1 can be assigned to O-H stretching mode for the adsorbed atmospheric moisture, and at about 1,500 cm−1, the C=O stretching mode arises from the absorption of atmospheric CO2 on the surface of the nanoparticles [19]. The main absorption band at 441.2 cm−1 is due to Zn-O stretching vibrations with a shift in their position as 460.0, 440.8, 437.6, 444.1 and 452.6 cm−1 for 0.05%, 0.1%, 0.2% and 0.5% Pd2+-doped ZnO nanocrystals, respectively. The change in the peak position of ZnO absorption band reflects that the Zn-O-Zn network is perturbed by the presence of Pd2+ in its environment [20, 21]. Therefore, the FTIR results also indicate that Pd is occupying the Zn position in the ZnO matrix as observed in XRD measurements.
Figure 5 shows the optical absorption spectra for TOPO-capped ZnO:Pd2+ nanocrystals at room temperature. The two prominent excitonic absorption peaks at 303 and 363 nm for ZnO nanoparticles were blue-shifted as compared with the bulk exciton absorption peak of ZnO (373 nm) which is due to the quantum size effects in nanocrystals [21, 22]. The variation of (αhv)2 versus photon energy (hv) for the synthesized nanocrystals is plotted in Figure 6. The optical gap (Eg) is estimated from the intercept of the extrapolated linear portion of the curve, and the values are summarised in Table 1. The value of optical gap increases to 3.23 eV (0.05% Pd) from 3.17 eV (for pure ZnO nanocrystals) and then decreases to 2.96 eV for 0.5% of Pd dopant. The structural modifications along with the improved crystallinity give rise to an increase in the Tauc's slopes or decrease the tailing; hence, there is an increase in the optical gap for a small amount of dopant in ZnO nanocrystals [23]. The small dopant also improves stoichiometry (i.e. R = 1 for 0.05% Pd2+), and further addition of dopant causes a deviation in stoichiometry due to the formation of oxygen vacancies in the synthesized nanocrystals. Thus, the red shift in the optical absorption edge can be explained on the basis of the increase in the defect density due to the formation of oxygen vacancies in doped ZnO nanoparticles and/or due to the effects of crystal-field splitting of 3d energy levels on the electronic transitions involving Pd2+ ions substituting the Zn2+ lattice sites.
The fluorescence spectroscopy was generally used to elucidate the nature of traps/vacancies formed with the incorporation of dopants. The type of vacancies formed depends upon the synthesis conditions and techniques used [24]. The room temperature luminescence spectra for colloidal solution of ZnO:Pd2+ nanocrystals at 350 nm of excitation energy are shown in Figure 7. Four luminescence peaks centred at 382, 413, 436 and 465 nm have been observed for the TOPO-capped ZnO nanocrystals. Generally, three emission bands are observed for ZnO nanocrystals [25]. The first band centred at 357 nm indicates band-to-band transition, the second band at 387 nm is for the radiative recombination of an electron and a hole, i.e. the excitonic emission [26] and the third emission at 420 nm may be attributed to the interstitial oxygen [25]. The luminescence at 435 nm may be due to the formation of Zn interstitial defect [27]. A blue luminescence at 465 nm which is normally not observed for ZnO nanocrystals may be due to impurities introduced during the course of preparation and can be attributed to the intrinsic defects such as O or Zn vacancies to valence band transition [28].
The incorporation of dopant significantly affects the shape and position of the luminescence spectrum with the formation of new bands at 528 and 581 nm for 0.05% Pd contents. This visible luminescence can either be ascribed to the formation of intrinsic oxygen vacancy defect states (Vo0, Vo+ and Vo2+) or Zn interstitials for ZnO nanocrystals [29]. The oxygen vacancies are located below the bottom of the conduction band (CB) as Vo0, Vo+ and Vo2+, from top to bottom. Thus, the green emission around 528 nm may be due to the recombination of a photogenerated hole with the singly ionised charged state of the specific defect [30, 31]. On the other hand, the shallow accepter levels at 0.3 eV above the top of the valence band are due to the zinc vacancy, and donor levels at 0.5 eV below the bottom of the CB are due to Zn interstitials which have been reported from the first principle calculations [30]. As the Pd2+ concentration increases, the intensity of the green emission also increases, indicating an enhancement in the singly ionised oxygen vacancy (Vo+) in doped ZnO nanocrystals. The detailed mechanism for defect-mediated green emission for ZnO nanoparticles has been discussed elsewhere [32]. Similarly, the luminescence band at approximately 581 nm can be due to the dominance of other intrinsic defects or the incorporation of Pd2+ into the ZnO lattice which perturbs its band structure to form a discrete energy level within the bandgap. Therefore, it seems that the dopant incorporation controls the various aspects of the recombination process and favours the radiative transitions from the oxygen vacancies/trap levels in the bandgap to give a broad visible luminescence for the Pd2+-doped ZnO nanocrystals.
Conclusions
The alkaline-activated wet chemical method has been successfully utilised for the synthesis of colloidal ZnO:Pd2+ nanocrystals at room temperature. It has been found that the dopant Pd2+ ions substitute for the Zn ions, without changing their wurtzite structure of the nanocrystals. The lattice parameters for the wurtzite phase of ZnO increase with the increase in dopant levels as 1.24 to 3.49 at.% of Pd. The optical gap initially increases with small doping (0.05% of Pd2+) and decreases with further addition of Pd2+ thereafter. Three characteristic luminescence bands at 357, 387 and 420 nm were observed for pure ZnO nanocrystals. New dominant bands centred at 528 and 581 nm for 0.5% of Pd2+-doped ZnO nanocrystals were observed. These results were attributed to the change in oxygen vacancy-related defects and/or formation of new trap states which effectively controls the luminescence properties of the doped nanocrystals which is an effective technique for modulating their optical properties.
Authors’ information
JK has completed her B.Sc. (NM), M.Sc. (Applied Physics) and M. Phil. (Physics) from the GND University, Amritsar, India. She is presently working for her Ph.D. in Physics (Material Science) at the same university. PK is working as an assistant professor in the Nanotechnology Research Centre, DAV Institute of Engineering and Technology, Kabir Nagar, Jalandhar, India. He received his B.Sc. (NM) from the Himachal Pradesh University, Shimla, India and his M.Sc. (Applied Physics) from the GND University, Amritsar, India. He also got post-doctoral fellowships as SRF (extended) and as research associate from CSIR New Delhi for 3 years. He obtained his Ph.D. in the field of material science, some thermal, optical and structural characterization of chalcogenide semiconductors from the same institute. He has published more than 25 international journal publications to his credit. His current research interest includes synthesis and characterization of metal oxide semiconductors, polymer nanocomposites, chalcogenide thin films etc. TSS has completed his Ph.D. in Physics from Indian Institute of Technology Delhi, New Delhi, India and is presently working as associate professor (Physics) at the University of Botswana, Gaborone, Botswana. His main Research interests are chalcogenide glasses, determination of optical constants of thin films, optical, electrical and structural properties of transparent conducting oxides, solar Energy materials, solar selective coatings, dye-sensitized solar cells and advanced material characterisation techniques. RT has completed his M.Sc. (Physics) from the Mysore University, Mysore, India and his Ph.D. (Physics) from the Indian Institute of Technology Delhi, New Delhi, India. He has also completed his post doctorate and worked as senior scientific officer at the Physics Department of the IIT Delhi, India. He has actively engaged in teaching/research with PG, Ph.D. and post doctorate students at the GND University, Amritsar, India. He has published more than 100 publications in reputable international journals. He has completed various research projects from the UGC, CSIR, DST etc. and is presently working as Emeritus professor (Physics) at the GND University, Amritsar, India.
References
Ozgur U, Alivov YI, Liu C, Take A, Reshchikov MA, Dogan S, Avrutin V, Cho SJ, Markoc H: A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98: 041301. 10.1063/1.1992666
Joo J, Kwon SG, Yu JH, Hyeon T: Synthesis of ZnO nanocrystals with cone, hexagonal cone, and rod shapes via non-hydrolytic ester elimination sol–gel reactions. Adv. Mater. 2005,17(15):1873. 10.1002/adma.200402109
Qu F, Santos DR Jr, Dantas NO, Monte AFG, Morais PC: Effects of nanocrystal shape on the physical properties of colloidal ZnO quantum dots. Physica E 2004, 23: 410. 10.1016/j.physe.2003.12.137
Kohls M, Schmidt T, Katschorek H, Spanhel L, Muller G, Mais N, Wolf A, Forchel A: A simple colloidal route to planar micropatterned Er@ZnO amplifiers. Adv. Mater. 1999,11(4):288. 10.1002/(SICI)1521-4095(199903)11:4<288::AID-ADMA288>3.0.CO;2-B
Tong YH, Liu YC, Lu SX, Dong L, Chen SJ, Xiao ZY: The optical properties of ZnO nanoparticles capped with polyvinyl butyral. J. Sol–Gel Sci. Tech. 2004, 30: 157.
Ohno H, Munekata H, Penney T, Molnar SV, Chang LL: Magnetotransport properties of p-type (In, Mn)As diluted magnetic III-V semiconductors. Phys. Rev. Lett. 1992, 68: 2664. 10.1103/PhysRevLett.68.2664
Schwartz DA, Norberg NS, Nguyen QP, Parker JM, Gamelin DR: Magnetic quantum dots: synthesis, spectroscopy, and magnetism of Co2+- and Ni2+-Doped ZnO Nanocrystals. J. Am. Chem. Soc. 2003, 125: 13205. 10.1021/ja036811v
Wang X, Zhang J, Zhu Z, Zhu J: Effect of Pd2+ doping on ZnO nanotetrapods ammonia sensor. Coll. Surf. A 2006, 276: 59. 10.1016/j.colsurfa.2005.10.026
Wang X, Zhang J, Zhu Z, Zhu J: Humidity sensing properties of Pd2+-doped ZnO nanotetrapods. Appl. Surf. Sci. 2007, 253: 3168. 10.1016/j.apsusc.2006.07.033
Al-Zaidi QG, Suhail AM, Al-azawi WR: Palladium-doped ZnO thin film hydrogen gas sensor. Appl. Phys. Res. 2011,3(1):89.
Wei S, Yu Y, Zhou M: CO gas sensing of Pd-doped ZnO nanofibers synthesized by electrospinning method. Mater. Lett. 2010,64(21):2284. 10.1016/j.matlet.2010.07.038
Chang CM, Hon MH, Leu IC: Improvement in CO sensing characteristics by decorating ZnO nanorod arrays with Pd nanoparticles and the related mechanisms. RSC Adv. 2012, 2: 2469. 10.1039/c2ra01016j
Gondal MA, Alzahrani AJ, Randhawa MA, Siddiqui MN: Morphology and antifungal effect of nano-ZnO and nano-Pd-doped nano-ZnO against Aspergillus and Candida. J. Environ. Sci. Health A 2012, 47: 1413.
Ramakanth K: Basic of Diffraction and its Application. New Delhi: I.K. International Publishing House; 2007.
Zhang J, Zhang Y, Xu KW, Ji V: General compliance transformation relation and applications for anisotropic hexagonal metals. Sol. Stat. Commun. 2006, 139: 87. 10.1016/j.ssc.2006.05.026
Cullity BD, Stock SR: Elements of X-ray Diffraction. New Delhi: Prentice Hall Publication; 2001.
Zak AK, Majid WHA, Abrishami ME, Yousefi R: X-ray analysis of ZnO nanoparticles by Williamson-Hall and size-strain plot methods. Sol. Stat. Sci. 2011, 13: 251. 10.1016/j.solidstatesciences.2010.11.024
Assadi MHN, Zhang Y, Zheng RK, Ringer SP, Li S: Structural and electronic properties of Eu- and Pd-doped ZnO. Nano. Res. Lett. 2011, 6: 357. 10.1186/1556-276X-6-357
Gondal MA, Drmosh QA, Yamani ZH, Saleh TA: Synthesis of ZnO2 nanoparticles by laser ablation in liquid and their annealing transformation into ZnO nanoparticles. Appl. Surf. Sci. 2009, 256: 298. 10.1016/j.apsusc.2009.08.019
Ahmed F, Kumar S, Arshi N, Anwar MS, Koo BH, Lee CG: Doping effects of Co2+ ions on structural and magnetic properties of ZnO nanoparticles. Microelect. Engg. 2011, 89: 129.
Dole BN, Mote VD, Huse VR, Purushotham Y, Lande MK, Jadhav KM, Shah SS: Structural studies of Mn doped ZnO nanoparticles. Curr. Appl. Phys. 2011, 11: 762. 10.1016/j.cap.2010.11.050
Sridevi D, Rajendran KV: Synthesis and optical characteristics of ZnO nanocrystals. Bull. Mater. Sci. 2009, 32: 165–168. 10.1007/s12034-009-0025-9
Kumar P, Thangaraj R, Sathiaraj TS: Electrical and optical study of phase transitions in thermally evaporated GeTe films. Phys. Stat. Solidi A 2011,208(4):838. 10.1002/pssa.201026537
Lakowicz JR: Principles of Fluorescence Spectroscopy, 4th printing. Heidelberg: Springer Science; 2006.
Modak S, Acharya S, Bandyopadhyay A, Karan S, Roy SK, Chakrabarti PK: Micro-structural investigations and paramagnetic susceptibilities of zinc oxide, europium oxide and their nanocomposites. J. Mag. Mag. Mater. 2010, 322: 283. 10.1016/j.jmmm.2009.09.030
Zhou J, Zhao F, Wang Y, Zhang Y, Yang L: Size-controlled synthesis of ZnO nanoparticles and their photoluminescence properties. J. Lumin. 2007, 122: 195.
Goswami N, Sharma DK: Structural and optical properties of unannealed and annealed ZnO nanoparticles prepared by a chemical precipitation technique. Physica E 2010, 42: 1675. 10.1016/j.physe.2010.01.023
Pereira AS, Peres M, Soares MJ, Alves E, Neves A, Monteiro T, Trindade T: Synthesis, surface modification and optical properties of Tb3+-doped ZnO nanocrystals. Nanotechnology 2006, 17: 834. 10.1088/0957-4484/17/3/037
Vanheusden K, Seager CH, Warren WL, Tallant DR, Voigt JA: Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68: 403. 10.1063/1.116699
Zhang SB, Wei SH, Zunger A: Intrinsic n-type versus p-type doping asymmetry and the defect physics of ZnO. Phys. Rev. B. 2001, 63: 075205.
Yang CL, Wang JN, Ge WK, Guo L, Yang SH, Shen DZ, Wang X, Zhang J, Zhu Z, Zhu J: Enhanced ultraviolet emission and optical properties in polyvinyl pyrrolidone surface modified ZnO quantum dots. J. Appl. Phys. 2001, 90: 4489. 10.1063/1.1406973
Sharma PK, Dutta RK, Pandey AC: Alteration of magnetic and optical properties of ultrafine dilute magnetic semiconductor ZnO: Co2+ nanoparticles. J. Coll. Inter. Sci. 2010, 345: 149. 10.1016/j.jcis.2010.01.050
Acknowledgements
Jasmeet Kaur is thankful to the head of the department for providing the necessary experimental facilities required to complete his M. Phil. dissertation work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JK perform the synthesis and took the necessary experimental data while PK supervised and prepared the manuscript. RT conducted the progress of the study and discussed the results in detail, and TSS performed the characterizations for the synthesized nanopowders. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kaur, J., Kumar, P., Sathiaraj, T.S. et al. Structural, optical and fluorescence properties of wet chemically synthesized ZnO:Pd2+ nanocrystals. Int Nano Lett 3, 4 (2013). https://doi.org/10.1186/2228-5326-3-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2228-5326-3-4