Abstract
ZnS nanoparticles were prepared by simple wet chemical precipitation method to study the effect of sulfur and trisodium citrate concentration on the particle growth of ZnS nanoparticles. Particle sizes were seen varied with the concentration of the sulfur and trisodium citrate. Compressive strain was observed only for a particular concentration of sulfur, and that sample was chosen for trisodium citrate experiments. Interestingly, the compressive strain was seen varied with the concentration of trisodium citrate and seen maximum for 2 M concentration of trisodium citrate. Further, interband transition was observed in the case of smaller crystallites prepared in the trisodium citrate. It is clearly observed from the FTIR analysis that the trisodium citrate acts as a stabilizer and capped the particles from further growth.
Similar content being viewed by others
Background
It is a well-known fact that the semiconductor nanoparticles exhibit novel optical, electronic, and physical properties than their bulk counterparts. When the size of the nanoparticles becomes less or comparable to the Bohr diameter of the exciton [1], various size quantization effects such as widening of band gap and formation of discrete orbitals come into the picture as explained by the Heisenberg uncertainty principle. Among the semiconducting nanoparticles, ZnS is one of the most important materials in the electronic/optoelectronic industry with prominent applications [2] in flat-panel displays [3], white light LEDs [4], electroluminescence devices [5], sensors [6], lasers [7], infrared windows [8], ultraviolet (UV) lasers [9], antireflection coating on solar cells [10], solar cells [11], and for investigating cellular interactions [12], for both in vivo-in vitro imaging applications [13], and memristor applications [14]. Basically, ZnS has negative conductivity [15, 16] and has a small exciton Bohr radius of about 2.5 nm [17]. Particle size reduction has a tremendous effect on the properties of the ZnS such as blueshift in the optical absorption spectrum, increased luminescence, enhanced oscillator strength, nonlinear optical effects, geometrical structure, chemical bonds, ionization potential, mechanical strength, melting point, etc. Furthermore, ZnS relates more popular ZnO in terms of atomic structure and chemical properties. Certain properties of ZnS are unique and advantageous compared to that of ZnO. ZnS has a larger band gap than that of ZnO (approximately 3.4 eV [17]), and therefore, it is more suitable for visible UV light-based devices such as sensors/photodetectors. However, nano-sized ZnS has not been investigated in much detail compared to ZnO nanomaterials.
This paper discusses the preparation of zinc sulfide nanocrystals by simple wet chemical precipitation method [18–21] and studies the effect of the concentration of sulfur and trisodium citrate on nanoparticle formation since trisodium citrate acts as surfactant and capping agent that can modify the surface of ZnS particles and prevents the growth of the particles.
Results and discussion
ZnS nanocrystalline powder synthesis by varying sulfur concentration
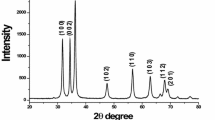
The prepared ZnS powder samples were analyzed by powder X-ray diffraction technique, scanning electron microscopy (SEM), energy dispersive X-ray (EDX), and UV-visible absorption spectrophotometer. The X-ray diffractogram of the prepared samples well correlates with the American Society for Testing and Materials (ASTM) card no. 77–2100 of cubic structure, and the diffraction peaks from the planes (111), (220), and (311) were noted in the diffractograms shown in Figure 1. All of the X-ray diffractogram peaks and the peak broadening confirmed that the prepared sample contains pure cubic ZnS nanocrystallites. Their crystallite size and the strain were obtained from the Williomson-Hall plot [22]. The crystallite size, strain, lattice parameters, and the volume of the crystals of each sample are shown in Table 1, and it is seen that the prepared ZnS powder consists of nanoparticles. Table 1 clearly shows the compressive strain for the samples ZnS0.1 and ZnS0.05, a property of nanoparticles.
EDX analysis of smaller crystallite samples were performed to find their elemental composition, and the results are shown in Figures 2, 3, and 4, where the elemental data are shown in the inset tables of Figures 2, 3, and 4. These data confirm that the prepared powder consist of only ZnS compound as seen from the X-ray diffractograms of those samples (Figure 1).
To obtain the visual image of the nanoparticles, these nanocrystalline powder samples were analyzed by SEM, and the micrographs are shown in Figures 2, 3, and 4. All of the SEM micrographs reveal that the nanograins are agglomerated due to the drying process, and the sizes of the particles are much larger than the values found from the XRD data. The discrepancy associated with the size of ZnS nanoparticles found from XRD and SEM may be because of the fact that SEM shows the lateral dimension of the particles whereas XRD gives the regularity in the atomic arrangement. Perhaps the lateral dimension is of the order of a few tens of nanometers, but the individual nanoparticle with regular atomic lattice extends only up to a few nanometers.
The study of optical absorption is important to understand the behavior of semiconductor nanocrystals since the fundamental property of semiconductors is the band gap that is the energy separation between the filled valence band and the empty conduction band. The optical excitation of electrons across the band gap is strongly allowed that produces an abrupt increase in absorption at the wavelength corresponding to the band gap energy. This feature of the optical spectrum is known as the optical absorption edge. High-quality ZnS structures have good ultraviolet optical properties at room temperature, and Figure 5 shows the optical absorption spectra of the prepared ZnS nanoparticles which is in the range of 800 to 200 nm. The absorption edge was obtained at shorter wavelengths compared to the absorption edge of the bulk materials due to the increase in band gap as a result of the quantum confinement effect as well as the discrete energy bands. Strong confinement is observed when the crystallite or particle size is smaller than the Bohr exciton radius of ZnS (approximately 2.5 nm [23]) as a result of this strong confinement absorption peaks which appear in the absorption spectra due to subband transitions, so that in this case, band gap can be found from the maximum of this absorption peak. However, when the crystallite size increases above the Bohr radius, the peak structure disappears and attains the normal interband transition spectrum [24] having an absorption edge at a lower wavelength than the bulk materials. In the bulk material or in thin films, the band gap can be found from the plot between (αhν)2 vs hν, whereas in the nanomaterials of sizes above Bohr radius, the band gap is obtained from the absorption edge, but when the nanomaterial size is less than Bohr exciton radius of the materials, it is obtained from the absorption peak.
When comparing the absorption spectrogram of each of the samples, it is well clear that the ZnS nanocrystalline powder prepared with 0.1 M of Na2S has the highest absorption intensity. Highest intensity of absorption means that there is a higher number of particles to absorb as well as this sample has the highest capacity to absorb the incident light. Each of the samples has the absorption around 280 nm (4.4 eV) which was blueshifted from 336 nm (3.7 eV) of the bulk ZnS [25]. The blueshifted absorption also confirms that the results obtained from the X-ray diffractograms and the SEM micrographs, that the powder samples are made up of nanocrystals, since the reduction in the size of particles leads to widening of the band gap and this widening is observable in the optical absorption spectra, where a blueshift is observed with the reduction in particle size. Brus [26] has proposed a theoretical model relating the effective band gap of a material with the particle size. The simple model predicting the variation of exciton energy with particle size is based on the effective mass approximation. The lowest excited state of the crystallite is assumed to be the ground state of an electron hole pair. The ground state energy of an exciton or the increase in effective band gap as a function of crystalline size is estimated as
where E g (r) is the band gap of the nanoparticle, E g,bulk is the bulk semiconductor band gap (approximately 3.68 eV for Cubic ZnS [25], approximately 3.9 eV for Wurtzite ZnS [25]), h is Planck's constant (6.6260 × 10−34 m2 kg/s or 4.1356 × 10−15 eV · s), ϵ r is the relative dielectric constant of the material (8.6 [25]), ϵo is the permittivity of free space, r is the radius of the particle, and m e and m h are the effective masses of the electron (approximately 0.3m e for ZnS [27]) and hole (approximately 0.6m e for ZnS [27]), respectively. Using the average crystallite size obtained from X-ray diffractograms, the band gap for each sample is calculated in Table 2. It is clear from Table 2 and Figure 6 that the band gap increases as the crystallite size decreases, and it is found that the sample ZnS0.1 possesses the smallest crystallites and has high absorption as well as higher band gap when compared with other samples, so that the stoichiometry of the ZnS0.1 was selected for further experiments.
Effect of trisodium citrate concentration
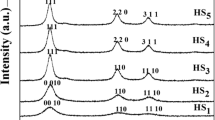
Here, trisodium citrate acts as a surfactant, and it allows easy spreading and lowering the surface tension of the solvent. Further, it acts as a microreactor for hosting the reaction and a steric stabilizer to inhibit the aggregation. X-ray diffractogram of the samples was compared with the standard ASTM cards of ZnS and ZnO, and it is found that all the diffractograms correlate with the ASTM card of ZnS 77–2100 (cubic). Their X-ray diffractograms are shown in Figure 7, and their broadened peaks reveal that the sample consisted of nano-sized crystallites. Interplanar spacing of the crystals was calculated and compared with ASTM data in the table given with Figures 8, 9, 10, 11, 12, and 13. Average crystallite sizes and strain were obtained from Williamson-Hall plot [22], and other crystal information is given in Table 3. It is seen from Table 3 that the ZnS crystals prepared in trisodium citrate have smaller crystal size when compared to the sample without this surfactant and formed various sizes of crystallites depending on the concentrations of the surfactants. Further, all of the samples show compressive strain, a property of nanomaterials. From all these experiments, ZnS prepared in 2 M of citrate has the lowest size of crystallites which is evident from Table 3. SEM micrographs of the samples shown in Figures 8, 9, 10, 11, 12, and 13 and the images reveal that well-dispersed particles of nanocrystallites have been formed as observed from the X-ray diffractogram. From this analysis, it is obvious that the ZnS particle growth can be controlled by the concentration of the surfactant sodium citrate.
Reported works of John et al. [28] and Yuan-yuan [20] on ZnS nanostructures without any surfactants show larger particles (12 and 35 nm, respectively) when compared to the work explained in this paper (1.46 to 2.25 nm). This comparison on particle size with the previous works [20, 28] also showed that surfactant sodium citrate has influence on the crystallization of ZnS nanocrystals.
Further, the sample prepared in 2 M of trisodium citrate was analyzed by transmission electron microscopy (TEM) by dispersing the powder in ethanol. Figure 14 shows the TEM, high-resolution transmission electron microscopy (HRTEM), and selected area electron diffraction (SAED) images of this sample, and it is explicitly shown that the particles are well below 10 nm. The polycrystalline diffraction rings of the SAED images are labeled to the corresponding planes according to the ASTM card of ZnS 77–2100 (cubic). The interplanar spacing of 0.23 nm found from HRTEM matches with the (111) planes which is smaller than the XRD data since the powder was dispersed in ethanol for TEM analysis that disintegrate the agglomerated particles and that further compresses the crystallites. The interplanar spacing (d) obtained from different analysis are compared in Table 4.
Optical absorption spectrum of the ZnS prepared in 2 M citrate is shown in Figure 15, and the appearance of the peak shows interband transitions due to the strong quantum confinement effect. The band gap of the sample is calculated and shown in Table 5 with the crystallite size. It is seen that the band gap calculated from the Brus equation is larger than the band gap obtained from the absorption spectra, which shows that the Brus equation cannot be expected to be quantitatively correct for very small particles [29], since the absorption in the case of small particles having size smaller than their exciton Bohr radius is due to the interband transition. Further, the agglomeration of the nanoparticles also has an effect in their absorption spectra.
Fourier transform infrared spectroscopy (FTIR) analysis was performed after dispersing the powder in ethanol to find the presence of trisodium citrate in the final product that helps to differentiate the properties of the pure ZnS nanocrystals with the ZnS nanocrystals with trisodium citrate. The FTIR spectra of the trisodium citrate, ZnS prepared without trisodium citrate, and ZnS prepared with citrate (sample C4) are shown in Figure 16, and the absorption bands are assigned to the respective vibrations as shown in Table 6. The FTIR spectra show that ZnS is covered with the surfactant trisodium citrate.
Conclusions
The preparation of zinc sulfide nanocrystals by simple wet chemical precipitation method is discussed here. It is found that the concentration of sulfur and trisodium citrate has tremendous effect on ZnS nanoparticle formation. The particle size was seen to depend on the concentration of trisodium citrate, and the dependence of the optical behavior on Bohr exciton radius of the ZnS nanoparticles was observed.
Methods
ZnS nanocrystalline powder synthesis by varying sulfur concentration
It is essential to find a suitable reagent concentration and stoichiometry for ZnS nanoparticles preparation, and therefore, it was decided to fix the concentration of Zn precursor (ZnCl2) and the surfactant trisodium citrate and to vary the concentration of the sulfur precursor (Na2S). These studies revealed the suitable Zn precursor and sulfur precursor concentrations for processing ZnS nanocrystallites.
ZnS samples were prepared by varying the sulfur concentration from 0.05 to 1.5 M, and the samples were named as ZnS0.05, ZnS0.1, ZnS0.5, ZnS1, and ZnS1.5. For this experiment, 50-ml stock solution of 1 M ZnCl2 and 0.5 M trisodium citrate was prepared in deionized (DI) water and concentration of Na2S was varied as shown in Table 7. Trisodium citrate solution (0.5 M) was added to each of the Na2S solution and stirred well for 1 hr at room temperature. Then, each of the 10.5-ml Na2S trisodium citrate solution was added dropwise using a burette to the 10-ml ZnCl2 solution while continuing the stirring (200 rpm) at room temperature. It was found that the color of the solution changed from a transparent solution to milky; then, a white precipitate was formed. The excess water with the dissolved by-product NaCl (ZnCl2 + Na2S → ZnS+2NaCl) was decanted, and the precipitate was washed in ethanol and then washed four times with deionized water. Since, ethanol can avoid the new formation of ZnS nanocrystals from undissolved Na2S and ZnCl2 (ZnCl2 partially dissolves in ethanol). After washing four times in DI water, the precipitate was again washed in ethanol and the excess ethanol was decanted, and then the precipitate was dried at 60°C in air since ethanol evaporates at around 50°C. After evaporation of the ethanol, a fine powder of ZnS was obtained and the color of the final powder was seen different for each sample as shown in Table 7.
Effect of trisodium citrate concentration
The effect of the concentrations of trisodium citrate on ZnS particle growth was studied by varying trisodium citrate concentration. Here, ZnCl2 and Na2S concentrations were fixed similar to the concentration of the sample ZnS0.1 in the ‘ZnS nanocrystalline powder synthesis by varying sulfur concentration’ section based on the results discussed in the ‘Results and discussion’ section. Stock solutions of 1 M ZnCl2 and 0.1 M Na2S were prepared as in the ‘ZnS nanocrystalline powder synthesis by varying sulfur concentration’ section, and then the solutions of trisodium citrate for various concentrations were prepared as shown in Table 8.
Each concentration of trisodium citrate solution was made under magnetic stirring for 10 min to make it a clear solution, and then each of the trisodium citrate solution was mixed with 10 ml Na2S solution. The stirring continued for 1 h until the solution became light blue, and then the Na2S-trisodium citrate solution was added drop by drop using a burette to 10 ml ZnCl2 solution while maintaining the stirring (about 200 rpm). It was seen that the solution became milky for each drop of Na2S-trisodium citrate, and after mixing all the Na2S-trisodium citrate solution, a white precipitate was obtained. Then, stirring was stopped to let the white precipitate settle at the bottom of the flask. The excess liquid was decanted, and then the settled white powder was washed in ethanol and then washed four times using DI water as explained in the ‘ZnS nanocrystalline powder synthesis by varying sulfur concentration’ section. After washing again with ethanol, the precipitate was taken into a heater which was maintained at about 60°C in air, and then finally, a white fine powder was obtained.
References
Suchita K, Gosavi SW, Urban J, Kulkarni SK: Nanoshell particles: synthesis, properties and applications. Curr. Sci. 2006, 91: 8.
Chen Z-G, Zou J, Wang D-W, Yin L-C, Liu G, Liu Q, Sun C-H, Yao X, Li F, Yuan X-L, Sekiguchi T, Lu GQ, Cheng H-M: Field emission and cathodoluminescence of ZnS hexagonal pyramids of zinc blende structured single crystals. Adv. Funct. Mater. 2009, 19: 484–490. 10.1002/adfm.200801178
Sakshi S, Dhoble SJ, Singh P, Ramrakhiani M: Fabrication of ZnS:Cu/PVA nanocomposite electroluminescence devices for flat panel displays. Adv. Materials Lett 2013,4(2):169–173.
Nizamoglu S, Demir HV: Excitation resolved color conversion of CdSe/ZnS core/shell quantum dot solids for hybrid white light emitting diodes. J. Appl. Phys. 2009, 105: 083112. 10.1063/1.3109151
Muller GO, Mach R, Selle B, Ohnishi H: Efficient ZnS-like alkaline earth sulfide electroluminescence. J. Crystal Growth 1990, 101: 999–1003. 10.1016/0022-0248(90)91121-6
Wang X, Xie Z, Huang H, Liu Z, Chen D, Shen G: Gas sensors, thermistor and photodetector based on ZnS nanowires. J. Mater. Chem. 2012, 22: 6845–6850. 10.1039/C2JM16523F
Sorokina IT, Sorokin E, Mirov S, Fedorov V, Badikov V, Panyutin V, Schaffers KI: Broadly tunable compact continuous-wave Cr2+:ZnS laser. Opt. Lett. 2002,27(12):1040–1042. 10.1364/OL.27.001040
Sumitomo Electric Industries, Ltd: Environmentally-resistant ZnS lens for far-infrared cameras (new products and techniques). Sei Tech. Rev. 2010, 71: 113–115.
Onodera C, Masaaki Y, Tadayoshi S, Tsunemasa T: Threshold current density in ZnS/MgBeZnS quantum well ultraviolet lasers. Optical Rev. 2010,17(3):159–160. 10.1007/s10043-010-0027-2
Gangopadhyay U, Kyunghea K, Dhungel SK, Mangalaraj D, Park JH, Yi J: Application of CBD zinc sulfide (ZnS) film to low cost antireflection coating on large area industrial silicon solar cell. Trans. Electrical and Electronic Materials 2004, 5: 1.
Bredol M, Matras K, Szatkowski A, Sanetra J, Prodi-Schwa A: P3HT/ZnS: A new hybrid bulk heterojunction photovoltaic system with very high open circuit voltage. Solar Energy Mater. Solar Cells 2009, 93: 662–666. 10.1016/j.solmat.2008.12.015
Hsieh M-F, Li JK-J, Lin JC-A, Huang S-H, Sperling RA, Parak WJ, Chang WH: Tracking of cellular uptake of hydrophilic CdSe/ZnS quantum dots/hydroxyapatite composites nanoparticles in MC3T3-E1 osteoblast cells. J. Nanosci. Nanotechnol. 2008, 8: 12.
Hussain S, Won N, Nam J, Bang J, Chung H, Kim S: One-pot fabrication of high-quality InP/ZnS (core/shell) quantum dots and their application to cellular imaging. Chem. Phys. Chem. 2009, 10: 1466–1470. 10.1002/cphc.200900159
Bhadra R, Singh VN, Mehta BR, Datta P: Studies on some aspects of ZnS nanocrystals for possible applications in electronics. Chalcogenide Lett. 2009,6(5):189–196.
Lan C, Gon J, Jiang Y, Ding Q: Fabrication of ZnS/SnO nanowire/nanosheet hierarchical nanoheterostructure and its photoluminescence properties. Comm: Cryst. Eng; 2012. 10.1039/C2CE26053K 10.1039/C2CE26053K
Shaban SM, Saeed NM, Raad MS, Shaban SM, Saeed NM, AL-Haddad RMS: Fabrication and study zinc sulfide schottky barrier detectors. Indian J. Sci. Technol. 2011, 4: 4.
Coleman VA, Jagadish C: Basic properties and applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications. Edited by: Jagadish C, Pearton S. Oxford: Elsevier; 2006:1–20.
Warada HC, Ghosh SC, Hemtanon B, Thanachayanont C, Dutt J: Luminescent nanoparticles of Mn doped ZnS passivated with sodium hexametaphosphate. Sci. Technol. Adv. Mater. 2005, 6: 296–301. 10.1016/j.stam.2005.03.006
Dong D-Q, Lan LI, Zhang X-S, Xu HAN, Hai-Ping AN: Effect of precursor molar ration of S2-/Zn2+ on particles size and photoluminescence of ZnS:Mn2+ nanocrystals. Chin. Phys. Lett. 2007,24(9):2661. 10.1088/0256-307X/24/9/055
Yuan-yuan SHE, Juan YANG, Ke-qiang QIU: Synthesis of ZnS nanoparticles by solid–liquid chemical reaction with ZnO and Na 2 S under ultrasonic. Trans. Nonferrous Met. Soc. China 2010, 20: s211-s215.
Zhao Z, Geng F, Cong H, Bai J, Cheng H-M: A simple solution route to controlled synthesis of ZnS submicrospheres, nanosheets and nanorods. Nanotechnology 2006, 17: 4731–4735. 10.1088/0957-4484/17/18/034
Williamson GK, Hall WH: X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1: 22. 10.1016/0001-6160(53)90006-6
Bhattacharjee B, Ganguli D, Iakoubovskii K, Stesmans A, Chaudhuri S: Synthesis and characterization of sol–gel derived ZnS: Mn2+ nanocrystallites embedded in a silica matrix. Bull. Mater. Sci. 2002,25(3):175–180. 10.1007/BF02711150
Sahu SN, Nanda KK: Nanostructure semiconductors:physics and applications. PINSA 2001,67(A, 1):103–130.
Wang J, Isshiki M: Wide-bandgap II–VI semiconductors: growth and properties. In Springer Handbook of Electronic and Photonic Materials. Edited by: Kasap S, Capper P. New York: Springer; 2007:325–342.
Brus LE: A simple model for the ionization potential, electron affinity and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79: 5566–5571. 10.1063/1.445676
Üzar N, Arikan MÇ: Synthesis and investigation of optical properties of ZnS nanostructures. Bull. Mater. Sci. 2011,34(2):287–292. 10.1007/s12034-011-0085-5
John R, Sasi Florence S: Optical, structural and morphological studies of bean-like ZnS nanostructures by aqueous chemical method. Chalcogenide Lett. 2010,7(4):269–273.
Prabhu RR, Abdul Khadar M: Characterization of chemically synthesized CdS nanoparticles. Pramana J. Phys. 2005,65(5):801–807. 10.1007/BF02704078
Acknowledgements
The authors thank STIC, Cochin University of Science and Technology, Cochin, Kerala and SAIF, North Eastern Hill University, Shillong, Meghalaya for XRD, SEM, FTIR, and TEM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AKT carried out the experiments, examined the results, and drafted the manuscript. AKAU coordinated the project and discussed the results. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Thottoli, A.K., Unni, A.K.A. Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles. J Nanostruct Chem 3, 56 (2013). https://doi.org/10.1186/2193-8865-3-56
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2193-8865-3-56