Abstract
Background
Inheritance of a mutation in either BRCA1 or BRCA2 accounts for approximately 5% of all breast cancer cases, but varies by country. Investigations into the contribution of BRCA mutations to breast cancer incidence in Greece have been, for the most part, limited by small sample sizes and by the use of cases selected for their family history of cancer. The aim of the current study was to estimate BRCA mutation frequencies in breast cancer patients unselected for family history.
Methods
To do so, we enrolled 127 unselected women with breast cancer from the Alexandra Hospital in Athens, Greece, a large public hospital in the city. Mutations in BRCA1 and BRCA2 were detected using a combination of techniques and were confirmed by direct sequencing. Two large genomic deletions were sought using mutation-specific assays. A detailed family history of cancer was obtained from each patient.
Results
We were able to successfully complete testing on samples from 127 women. Among these, six mutations were identified (four in BRCA1 and two in BRCA2) representing 4.7% of the total or 9.5% of cases diagnosed before age forty. None of the mutation carriers had a family history of breast or ovarian cancer. Three of the four BRCA1 mutations were in exon 20: two were a G5331A mutation and the third was a 3.2 kb deletion. The fourth BRCA1 mutation was the 3819delGTAAA in exon 11. The two BRCA2 mutations were in exon 11 (3782del10 and 4512insT).
Conclusions
The G5331A mutation in BRCA1 appears to be a founder mutation in the Greek population.
Similar content being viewed by others
Background
The inheritance of a deleterious mutation in one of the two breast cancer susceptibility genes, BRCA1 and BRCA2, is associated with a high lifetime risk of breast cancer, currently estimated at 80% by the age of 70 [1–3]. Deleterious mutations in both genes also increase the lifetime risks of ovarian cancer and predispose men and women to a range of other malignancies [4–6]. Factors that aid in predicting whether a mutation has been inherited by a woman with breast cancer include the number of breast and/or ovarian cancer patients in the family, the age of diagnosis, her ancestry and various pathological features of the breast cancer [7, 8].
In North America, Europe, Israel and Australia, women considered to be at high-risk due to a significant family history of breast cancer may undergo molecular testing for mutations in BRCA1 and BRCA2. Genetic screening provides the opportunity for unaffected women who are identified as a carrier of a deleterious mutation to consider various risk-reducing options (e.g., prophylactic mastectomy and/or oophorectomy, tamoxifen) [9], intensive screening/surveillance (e.g., annual MRI, mammography) [10], and for women with cancer, the opportunity for individualized cancer therapy [8]. Exploring the prevalence of BRCA mutations in Greek patients will help establish whether the implementation of genetic services is warranted in that country.
A number of studies have evaluated the BRCA mutation status of breast cancer patients in Greece [11–15]. These studies have found that the common Ashkenazi Jewish mutation, 5382insC and G5331A [16], are possible founder mutations in the Greek population. Although these studies provide evidence for a role of genetics in the etiology of breast cancer in Greece, they have been limited by their small study population and the use of cases selected for a family history of cancer. Evaluation of a large Greek population of unselected cases will provide more accurate and unbiased evidence for risk associated with BRCA mutations and may lead to the detection of additional founder mutations. We performed mutation analysis of BRCA1 and BRCA2 on 127 unselected patients with breast cancer in Athens, Greece.
Methods
Patient Population
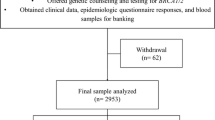
We conducted a study of unselected breast cancer patients, diagnosed at or before age 80, in Athens, Greece from February 10, 2006 to April 26, 2008. Patients were recruited from a single large public hospital in Athens (Alexandra Hospital). The study population consisted of patients undergoing treatment for a recent diagnosis of breast cancer. Unselected, newly diagnosed breast cancer cases were identified from the breast cancer clinic at Alexandra Hospital. Cases were approached about the study during their visit to the hospital. The study was introduced and described to the patient by the breast surgeon or research assistants. If the patient agreed to participate, she was provided a written description of the study, including a consent form. The patient was informed of the implications of genetic testing. At the time of the clinic appointment, a risk factor questionnaire was completed and a family history was recorded. A blood sample was obtained for DNA extraction. Mutation analysis took place in the laboratory of Dr. Steven Narod (Toronto, Canada). The institutional review boards at the participating centers approved the protocol and all of the women in the study provided written informed consent.
Patients were identified through the pathology and surgical records of the breast cancer service and were invited to participate through a special invitation. Of these, 50 were above the age of 80 and were ineligible. In total, 127 women were included in this study. On average, 3.7 years had elapsed between the date of diagnosis and the date of interview. All participants were interviewed in person for their family history of cancer, with specific reference to a history of breast or ovarian cancer. Tumor histology, tumor size, lymph node involvement and grade were abstracted from the medical records. Questionnaires on family histories and lifestyle factors were completed for all 127 cases.
All samples were screened for six common alterations, and two large genomic deletions, based on previous literature [11–17]. The common BRCA1 5382insC mutation was detected using a multiplex PCR reaction as previously described [17]; however, two separate screening assays were designed using restriction enzymes for two other BRCA1 mutations G5331A and C5370CT. All mutations were confirmed through direct sequencing. The G5586A mutation, which leads to exon 23 skipping [13], was detected by direct sequencing on all samples, and previously designed TaqMan Copy Number Variation Assays (ABI) were used for the large deletions del3.2kb, and del4429ins5 both in BRCA1. Assay ID numbers are available upon request. Protein truncation testing (PTT) was used to detect BRCA2 mutations 2024del5 and 4147delG in exons 10 and 11, respectively. PTT was performed using the TNT™ rabbit reticulocyte lysate system (Promega), involving [35S]methionine/cysteine (New England Nuclear) for protein detection. Aberrant and mutation positive bands were sequenced and confirmed by direct sequencing.
Results
A total of 127 breast cancer patients were tested for BRCA1 and BRCA2 mutations using a combination of laboratory techniques. The mean age of the patients at diagnosis was 50.8 years (range 19.3-78.2 years) and the average age at interview was 54.4 years (range 24.1-78.5). Sixteen percent of the patients were diagnosed before the age of 40 (n = 21) and 49% were diagnosed before the age of 50 (n = 62). Seventeen (13%) of the patients had a family history of breast or ovarian cancer and 13 (10%) of the patients had first-degree relative diagnosed with breast or ovarian cancer.
Overall, six mutations were identified (4.7%; 95%CI 0.018-0.10), including four in BRCA1 (3.1%) and two in BRCA2 (1.6%) (Table 1). Three of the four BRCA1 mutations were in exon 20: two were the G5331A mutation and the third was the 3.2 kb deletion. The fourth BRCA1 mutation was 3819delGTAAA in exon11. Both the BRCA2 mutations were in exon 11 (3782del10 and 4512insT). A mutation was seen in two of 21 patients diagnosed with breast cancer before age 40 (9.5%), in two of 46 patients diagnosed between ages of 40 and 50 (4.3%) and in two of 60 cases diagnosed above age 50 (3.3%). One of the six mutation carriers had a family history of breast or ovarian cancer and a first-degree relative diagnosed with breast or ovarian cancer (16.7%).
Discussion
The aim of the current study was to estimate the prevalence of BRCA mutations in a series of unselected female breast cancer cases from Greece. We identified a deleterious BRCA1 or BRCA2 mutation in 4.7% of the women with breast cancer. The most common mutation was the G5331A mutation, accounting for 1.6% of the cancers diagnosed and 33% of all mutations. Four additional mutations were identified; two in BRCA1 and two in BRCA2. Other studies of unselected populations have reported relatively low BRCA mutation frequencies (range: BRCA1 0-7%; BRCA2 1-3%) (reviewed in [18]).
One other hospital-based study has evaluated the frequency of BRCA1 (but not BRCA2) mutations among unselected breast cancer cases in Greece [19]. The authors evaluated the prevalence of four specific BRCA1 mutations. They previously reported that these account for 54% of all mutations detected in both genes [19]. They found that 26 of the 987 patients (2.6%) carried one of four mutations in BRCA1: 5382insC (1.3%), 5331G>A (0.4%), and two large genomic rearrangements involving deletions of exons 20 (0.3%) and 24 (0.6%). 5382insC has been previously described in numerous populations while the latter three mutations have only been described in Greek populations. Further, 14 of the 26 (54%) carriers had an early age of diagnosis (<40 years) and 10% of all the early-onset patients carried one of the four mutations. Given that the original data was based on the testing of 287 Greek families with a history of breast and/or ovarian cancer, this was not a study of unselected patients.
Nearly all the other prior studies evaluating BRCA mutation frequencies among patients in Greece have included women with familial breast and/or ovarian cancer (i.e., those with a family history) while the women in the current study were unselected for a personal or family history of cancer [11–16]. In the most comprehensive of these studies, the authors identified 26 BRCA mutations (15 BRCA1 and 11 BRCA2) in 287 Greek breast/ovarian cancer families using dHPLC followed by direct sequencing [16]. Four BRCA1 mutations accounted for more than half of the identified mutations, while the remaining 22 mutations were a mixture of unique mutations occurring at a low frequency suggesting a genetically heterogenous population [20]. The 5382insC and G5331A mutations in exon 20 of BRCA1 accounted for 46% of the families. We did not detect the 5382insC frameshift mutation in our study population. Although the 5382insC mutation is known as a founder mutation in the Ashkenazi Jewish population, Hamel et al. recently estimated that this mutation likely arose approximately 1,800 years ago in Scandinavia or northern Russia and infiltrated the Ashkenazi Jewish population 400-500 years ago [21]. Further, the authors concluded that this is likely the most common mutation in many European countries.
Relatively few BRCA mutations account for BRCA-associated cancers among homogenous populations such as Iceland and Poland, while more heterogeneous populations such as in Canada or the United States tend to display a broad mutation spectrum [18, 22, 23]. Founder mutations are specific mutations that appear repeatedly in ethnically defined groups because of a shared common ancestry. Such mutations have been identified in Icelandic [24], Polish [25], French-Canadian [26], and Ashkenazi Jewish [27], among other, populations. If genetic testing is to be offered to high-risk individuals, it is more economical to screen for founder mutations rather than comprehensive genetic testing which is considerably more costly.
Based on our and prior findings [14, 16, 28], it appears that G5331A mutation in BRCA1 may be a founder mutation in the Greek population. Previous studies indicate that the 5382insC frameshift [11, 13, 14, 16, 21] is likely a founder mutation as well, although this was not the case in the current study. In addition to these two mutations, many others reported several novel mutations, deletions or genomic rearrangements as well as unclassified variants and [11, 12, 15, 16, 19] suggesting a genetically heterogeneous population.
A major strength of the current study were the inclusion of unselected breast cancer cases thus allowing for an accurate estimation of the proportion of cases due to BRCA mutations. Further, genetic testing for BRCA1 and BRCA2 is highly sensitive [29]. Nonetheless, we were limited by the relatively small sample size (n = 127). The majority of the aforementioned studies were limited by the inclusion of familial cases, the relatively small sample sizes (n range 25-287) and the screening of one rather than both BRCA genes.
Conclusions
Inheritance of a deleterious BRCA mutation is one of the most important predictors of an individual's risk of developing breast cancer. Given that breast cancer is the most commonly diagnosed malignancy among women in Greece, it is of value to identify those at a high-risk of developing the disease. Genetic screening provides the opportunity for women identified as carriers of a deleterious mutation to consider various prevention strategies (e.g., prophylactic mastectomy and/or oophorectomy, tamoxifen) [9] or intensive screening (e.g., annual MRI, mammography) [10] to help lessen the burden of this disease.
Abbreviations
- BRCA1 :
-
breast cancer susceptibility gene 1
- BRCA2 :
-
breast cancer susceptibility gene 1.
References
Chen S, Parmigiani G: Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 2007, 25: 1329–1333. 10.1200/JCO.2006.09.1066
Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE: Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet 1994, 343: 692–695. 10.1016/S0140-6736(94)91578-4
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et al.: Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003, 72: 1117–1130. 10.1086/375033
Robson ME: Clinical considerations in the management of individuals at risk for hereditary breast and ovarian cancer. Cancer Control 2002, 9: 457–465.
Thompson D, Easton DF: Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002, 94: 1358–1365. 10.1093/jnci/94.18.1358
Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium J Natl Cancer Inst 1999, 91: 1310–1316. 10.1093/jnci/91.15.1310
Narod S, Ford D, Devilee P, Barkardottir RB, Eyfjord J, Lenoir G, Serova O, Easton D, Goldgar D: Genetic heterogeneity of breast-ovarian cancer revisited. Breast Cancer Linkage Consortium. Am J Hum Genet 1995, 57: 957–958.
Narod SA, Foulkes WD: BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 2004, 4: 665–676. 10.1038/nrc1431
Metcalfe KA, Snyder C, Seidel J, Hanna D, Lynch HT, Narod S: The use of preventive measures among healthy women who carry a BRCA1 or BRCA2 mutation. Fam Cancer 2005, 4: 97–103. 10.1007/s10689-005-4215-3
Warner E, Causer PA: MRI surveillance for hereditary breast-cancer risk. Lancet 2005, 365: 1747–1749. 10.1016/S0140-6736(05)66520-8
Konstantopoulou I, Kroupis C, Ladopoulou A, Pantazidis A, Boumba D, Lianidou ES, Petersen MB, Florentin L, Chiotellis E, Nounesis G, et al.: BRCA1 mutation analysis in breast/ovarian cancer families from Greece. Hum Mutat 2000, 16: 272–273. 10.1002/1098-1004(200009)16:3<272::AID-HUMU17>3.0.CO;2-4
Armakolas A, Ladopoulou A, Konstantopoulou I, Pararas B, Gomatos IP, Kataki A, Konstadoulakis MM, Stathopoulos GP, Markopoulos C, Leandros E, et al.: BRCA2 gene mutations in Greek patients with familial breast cancer. Hum Mutat 2002, 19: 81–82.
Ladopoulou A, Kroupis C, Konstantopoulou I, Ioannidou-Mouzaka L, Schofield AC, Pantazidis A, Armaou S, Tsiagas I, Lianidou E, Efstathiou E, et al.: Germ line BRCA1 & BRCA2 mutations in Greek breast/ovarian cancer families: 5382insC is the most frequent mutation observed. Cancer Lett 2002, 185: 61–70. 10.1016/S0304-3835(01)00845-X
Belogianni I, Apessos A, Mihalatos M, Razi E, Labropoulos S, Petounis A, Gaki V, Keramopoulos A, Pandis N, Kyriacou K, et al.: Characterization of a novel large deletion and single point mutations in the BRCA1 gene in a Greek cohort of families with suspected hereditary breast cancer. BMC Cancer 2004, 4: 61. 10.1186/1471-2407-4-61
Kataki A, Gomatos I, Pararas N, Armakolas A, Panousopoulos D, Karantzikos G, Voros D, Zografos G, Markopoulos C, Leandros E, Konstadoulakis M: Identification of germline BRCA1 and BRCA2 genetic alterations in Greek breast cancer moderate-risk and low-risk individuals--correlation with clinicopathological data. Clin Genet 2005, 67: 322–329. 10.1111/j.1399-0004.2004.00400.x
Konstantopoulou I, Rampias T, Ladopoulou A, Koutsodontis G, Armaou S, Anagnostopoulos T, Nikolopoulos G, Kamakari S, Nounesis G, Stylianakis A, et al.: Greek BRCA1 and BRCA2 mutation spectrum: two BRCA1 mutations account for half the carriers found among high-risk breast/ovarian cancer patients. Breast Cancer Res Treat 2008, 107: 431–441. 10.1007/s10549-007-9571-2
Kuperstein G, Foulkes WD, Ghadirian P, Hakimi J, Narod SA: A rapid fluorescent multiplexed-PCR analysis (FMPA) for founder mutations in the BRCA1 and BRCA2 genes. Clin Genet 2000, 57: 213–220.
Fackenthal JD, Olopade OI: Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer 2007, 7: 937–948. 10.1038/nrc2054
Armaou S, Pertesi M, Fostira F, Thodi G, Athanasopoulos PS, Kamakari S, Athanasiou A, Gogas H, Yannoukakos D, Fountzilas G, Konstantopoulou I: Contribution of BRCA1 germ-line mutations to breast cancer in Greece: a hospital-based study of 987 unselected breast cancer cases. Br J Cancer 2009, 101: 32–37. 10.1038/sj.bjc.6605115
Jobling MA, Tyler-Smith C: The human Y chromosome: an evolutionary marker comes of age. Nat Rev Genet 2003, 4: 598–612.
Hamel N, Feng BJ, Foretova L, Stoppa-Lyonnet D, Narod SA, Imyanitov E, Sinilnikova O, Tihomirova L, Lubinski J, Gronwald J, et al.: On the origin and diffusion of BRCA1 c.5266dupC (5382insC) in European populations. Eur J Hum Genet 2011, 19: 300–306. 10.1038/ejhg.2010.203
Neuhausen SL: Founder populations and their uses for breast cancer genetics. Breast Cancer Res 2000, 2: 77–81. 10.1186/bcr36
Ferla R, Calo V, Cascio S, Rinaldi G, Badalamenti G, Carreca I, Surmacz E, Colucci G, Bazan V, Russo A: Founder mutations in BRCA1 and BRCA2 genes. Ann Oncol 2007,18(Suppl 6):vi93–98.
Tulinius H, Olafsdottir GH, Sigvaldason H, Arason A, Barkardottir RB, Egilsson V, Ogmundsdottir HM, Tryggvadottir L, Gudlaugsdottir S, Eyfjord JE: The effect of a single BRCA2 mutation on cancer in Iceland. J Med Genet 2002, 39: 457–462. 10.1136/jmg.39.7.457
Gorski B, Byrski T, Huzarski T, Jakubowska A, Menkiszak J, Gronwald J, Pluzanska A, Bebenek M, Fischer-Maliszewska L, Grzybowska E, et al.: Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am J Hum Genet 2000, 66: 1963–1968. 10.1086/302922
Tonin PN, Mes-Masson AM, Futreal PA, Morgan K, Mahon M, Foulkes WD, Cole DE, Provencher D, Ghadirian P, Narod SA: Founder BRCA1 and BRCA2 mutations in French Canadian breast and ovarian cancer families. Am J Hum Genet 1998, 63: 1341–1351. 10.1086/302099
Tonin P, Serova O, Lenoir G, Lynch H, Durocher F, Simard J, Morgan K, Narod S: BRCA1 mutations in Ashkenazi Jewish women. Am J Hum Genet 1995, 57: 189.
Anagnostopoulos T, Pertesi M, Konstantopoulou I, Armaou S, Kamakari S, Nasioulas G, Athanasiou A, Dobrovic A, Young MA, Goldgar D, et al.: G1738R is a BRCA1 founder mutation in Greek breast/ovarian cancer patients: evaluation of its pathogenicity and inferences on its genealogical history. Breast Cancer Res Treat 2008, 110: 377–385. 10.1007/s10549-007-9729-y
Berry DA, Iversen ES Jr, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, Lerman C, Watson P, Lynch HT, Hilsenbeck SG, et al.: BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol 2002, 20: 2701–2712. 10.1200/JCO.2002.05.121
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SAN and JK conceived the study. CD and AA participated in the design of the study and in the supervision of the accrual of study participants. CK interviewed the participants and collected information. RR and SZ conducted the mutation analysis. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Koumpis, C., Dimitrakakis, C., Antsaklis, A. et al. Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Greece. Hered Cancer Clin Pract 9, 10 (2011). https://doi.org/10.1186/1897-4287-9-10
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1897-4287-9-10