Abstract
Background
The rapid adoption of image-guidance in prostate intensity-modulated radiotherapy (IMRT) results in longer treatment times, which may result in larger intrafraction motion, thereby negating the advantage of image-guidance. This study aims to qualify and quantify the contribution of image-guidance to the temporal dependence of intrafraction motion during prostate IMRT.
Methods
One-hundred and forty-three patients who underwent conventional IMRT (n=67) or intensity-modulated arc therapy (IMAT/RapidArc, n=76) for localized prostate cancer were evaluated. Intrafraction motion assessment was based on continuous RL (lateral), SI (longitudinal), and AP (vertical) positional detection of electromagnetic transponders at 10 Hz. Daily motion amplitudes were reported as session mean, median, and root-mean-square (RMS) displacements. Temporal effect was evaluated by categorizing treatment sessions into 4 different classes: IMRTc (transponder only localization), IMRTcc (transponder + CBCT localization), IMATc (transponder only localization), or IMATcc (transponder + CBCT localization).
Results
Mean/median session times were 4.15/3.99 min (IMATc), 12.74/12.19 min (IMATcc), 5.99/5.77 min (IMRTc), and 12.98/12.39 min (IMRTcc), with significant pair-wise difference (p<0.0001) between all category combinations except for IMRTcc vs. IMATcc (p>0.05). Median intrafraction motion difference between CBCT and non-CBCT categories strongly correlated with time for RMS (t-value=17.29; p<0.0001), SI (t-value=−4.25; p<0.0001), and AP (t-value=2.76; p<0.0066), with a weak correlation for RL (t-value=1.67; p=0.0971). Treatment time reduction with non-CBCT treatment categories showed reductions in the observed intrafraction motion: systematic error (Σ)<0.6 mm and random error (σ)<1.2 mm compared with ≤0.8 mm and <1.6 mm, respectively, for CBCT-involved treatment categories.
Conclusions
For treatment durations >4-6 minutes, and without any intrafraction motion mitigation protocol in place, patient repositioning is recommended, with at least the acquisition of the lateral component of an orthogonal image pair in the absence of volumetric imaging.
Similar content being viewed by others

Background
Intensity-modulated radiotherapy (IMRT) techniques for prostate cancer facilitate safe dose escalation through maximization of the therapeutic ratio with proven benefits on local recurrence and biochemical control rates [1, 2]. Given the very conformal nature of IMRT treatment plans, accurate patient positioning utilizing image-guidance systems is of the utmost importance [3]. Commercial availability of real-time or near-real-time monitoring devices [4–7] has permitted detailed evaluation of intrafraction prostate motion. Consequentially, it is now known that for a typical radiotherapy fraction, motion is largest in the antero-posterior and cranio-caudal axes [8, 9], and that the probability of intrafraction motion has a temporal dependence [5, 10, 11]. The latter finding is vital, as IMRT treatment times are typically longer than their conventional counterparts.
Curtis et al. [7] recently showed that with volumetric image-guidance every 4 minutes, a 3-mm margin is sufficient to account for intrafraction motion errors in the absence of positional correction measures based on real-time continuous tracking of the prostate. Shelton et al. [6], however, showed that an optimized workflow with faster treatment techniques, such as intensity-modulated arc therapy (IMAT), allows for a significant decrease in prostate intrafraction motion errors. Therefore, the current study aims to evaluate the temporal dependence of intrafraction motion of the prostate gland on image-guided IMRT techniques in the absence of positional correction measures based on real-time continuous tracking of the prostate. We hypothesize that the addition of image-guidance on prostate IMRT results in significantly longer treatment times, resulting in significant intrafraction motion error.
Methods
Patient population and treatment planning
The current retrospective analysis was approved by our Institutional Review Board (IRB), with patient informed consent waiver. Medical records of patients that underwent prostate IMRT or IMAT between September 2007 and July 2011 were reviewed. A cohort of 143 patients (mean/median age: 68/67; range: 51–87) with histologically confirmed clinical stage I-III prostate adenocarcinoma formed the basis of the current analysis.
Simulation and radiotherapy planning techniques have been reported previously [12]. In brief, simulation computed tomography (CT) images were acquired in the treatment position with the patient supine and transferred to a three-dimensional dosimetric planning platform (Pinnacle3 v7.6, Philips Medical Systems, Andover, MA or Eclipse v8.6/8.9, Varian Medical Systems, Inc., Palo Alto, CA, USA) for structure segmentation and treatment planning. Seven to 9-field step-and-shoot or sliding window IMRT plans (n=67) were computed for a prescribed dose of 70 Gy (2.5 Gy/28 fractions) or 78 Gy (2 Gy/39 fractions). Two sequential-arc (358° each) IMAT plans (n=76) were computed for a prescribed dose of 70 Gy (2.5 Gy/28 fractions).
Daily target localization and intrafraction motion analysis
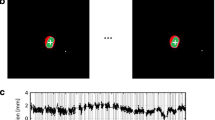
Patient setup procedures have also been previously described [12]. In brief, daily localization was based on electromagnetic transponder detection, validated at least weekly by volumetric cone-beam CT (CBCT; Varian Medical Systems, Palo Alto, CA, USA). Intrafraction motion of the prostate gland was monitored in real-time with 1/10th s update of the lateral (LR/X), vertical (AP/Y), and longitudinal (SI/Z) displacement of the localized target isocentre. Sustained deviations >4 mm in any translational direction prompted corrective action while transient deviations lasting <1 second were ignored. Tracking data points, obtained immediately post electromagnetic transponder localization and up to 30 s after dose administration, were used for the current evaluation. Tracking data with unusually large displacement of the target isocentre at the terminal portion of the tracking session (due to motion of the patient support assembly or treatment couch prior to discontinuation of real-time tracking) were excluded. Records of treatment fractions that were corrupted (i.e., containing no tracking data) were also excluded.
Intrafraction positional error estimation
Tracking sessions were separated into 4 distinct “treatment categories:” IMRTc (n=1226; IMRT sessions with setup based on electromagnetic transponders only), IMRTcc (n=864; IMRT sessions with setup based on electromagnetic transponders plus CBCT verification), IMATc (n=1461; IMAT sessions with setup based on electromagnetic transponders only) and IMATcc (n=586; IMAT sessions with setup based on electromagnetic transponders plus CBCT verification). Digital tracking data and associated time stamps were downloaded to Microsoft Excel (2007; Microsoft, Redmond, WA, USA) for processing. The root-mean-square (RMS) distance was calculated for each time point.
For reporting the individual directional components of target motion, the absolute values of motion displacements were used. For the purpose of this study, +X, +Y, and +Z coordinates defined displacement to the left, posterior, and superior directions from isocentre, respectively. Daily directional components were synchronized at each time point, and the population-based average computed for each treatment category was used to evaluate motion trend over time. Daily treatment couch corrections for sustained deviations >4 mm were reported.
To summarize the large amount of data points for each daily session into an analyzable endpoint for the entire cohort, the median observed absolute displacement was used. Furthermore, for each treatment category, population-based mean (M), systematic (Σ), and random (σ) errors were computed for each motion direction. Population-based effect of treatment duration on intrafraction motion amplitude was also assessed by computing displacement probabilities as a function of displacement and time.
Statistical analysis
Correlations between outcome (dependent) variable and effect variables were analyzed using a linear mixed effects model. The primary outcome of interest was intrafraction motion (median LR, median SI, median AP, and median RMS) and the measured covariate was session treatment time. Subject specificity served as random effect while the fixed (main) effect was the treatment category (IMRTc, IMRTcc, IMATc, and IMATcc). Heterogeneity between treatment categories was taken into account. Hypothesis testing for all pair-wise comparisons were adjusted for the family-wise errors across multiple comparisons at the 0.05 level using the Bonferroni correction. All statistical analyses were performed using SAS statistical software (version 9.0; SAS Inc, Cary, NC, USA).
Results
Magnitude and duration of prostate displacement
Figure 1 summarizes the duration of treatment sessions per treatment category. The observed mean/median session duration were 4.15/3.99 min (IMATc), 5.99/5.77 min (IMRTc), 12.74/12.19 min (IMATcc), and 12.98/12.38 min (IMRTcc); overall range: 1.8 min (IMRTc) to 51.8 min (IMRTcc). Pair-wise comparison between treatment categories showed significant differences (p<0.0001), except for IMRTcc vs. IMATcc (p>0.05). Although not the original intent of the current study, a comparison between CBCT (least square mean = 12.88 min) and non-CBCT (least square mean = 4.99 min) categories also showed a statistically significant difference (least square mean difference = 7.90 min; p<0.0001).
Box-and-whisker plots of the distribution of treatment session times for each treatment category. Whiskers denote the nearest values <1.5 times the interquartile range (IQR), open circles beyond the whisker lines (o) indicate individual outliers (<3.0 times the IQR), and stars (*) signify extreme values (>3.0 times the IQR).
The probability of isocentre displacement from reference position is presented in Figure 2. For the purpose of histogram analysis, displacement from the reference position was divided into 0.25-mm increments. Movement was most prominent in the SI and AP dimensions in all four tracking categories. Table 1 summarizes the fraction of time the treatment isocentre deviated from its reference position by ≥0 mm, ≥1 mm, ≥2 mm, ≥3 mm, or ≥4 mm in the LR, AP, and SI directions. Longitudinal (SI) motion for treatment categories that included volumetric imaging showed the greatest variability: 17.6% vs. 11.1% of IMRT sessions and 22.2% vs. 9.8% of IMAT sessions had displacement values ≥2 mm. On the other hand, LR motion showed very little variation, again with the most variability observed with the use of volumetric image-guidance. Deviations >5 mm were rare in any dimension secondary to the use of a 4-mm action level corrective intervention protocol. Overall, Table 2 indicates a posterior and inferior prostate drift, with the inferior drift more pronounced than the posterior.
Intra-treatment table adjustments
Treatment couch adjustments occurred 601 times out of 4137 treatment sessions. The incidence of required intra-treatment patient support assembly adjustment was as low as 6.6% (IMATc) to 8.3% (IMRTc) and as high as 27.2% (IMRTcc) to 28.3% (IMATcc). The incidence of treatment couch adjustment was 0.5% in the LR, 30.3% in the AP, and 69.2% in the SI directions, respectively. As expected, the accumulated incidence of table position adjustment was significantly higher for the techniques with longer session duration (IMRTcc and IMATcc) compared with their shorter equivalent (IMRTc and IMATc) in all motion directions. Furthermore, treatment couch adjustments, on average, occurred as early as 2.4 minutes into a tracking session, with the vast majority of table adjustments for the longer treatment sessions occurring onwards of 4.5 minutes (Table 2).
Prostate displacement as a function of time
Median motion difference amongst treatment categories in the LR direction showed significant difference between IMRTc vs. IMATc (p=0.0003) and between IMATc vs. IMATcc (p=0.0004). In the AP direction, significant difference was observed between IMRTc vs. IMRTcc (p=0.0176) and between IMRTc vs. IMATcc (p=0.0349). In the SI direction, significant difference was observed between IMRTc vs. IMATcc (p=0.0003) and between IMATc vs. IMATcc (p=0.0021). When adjusted for treatment time, significant difference was observable only between IMRTc and IMATc (p=0.0024) in the LR direction. Notwithstanding, median motion difference between CBCT and non-CBCT tracking sessions showed strong correlation with time for RMS (t-value=17.29; p<0.0001), SI (t-value=−4.25; p<0.0001) and AP (t-value=2.76; p<0.0066), and weak correlation for LR motion (t-value=1.67; p=0.0971).
The mean displacement in the LR and AP directions trended toward 0 mm for the majority of the sampled time points (Figure 3); relating to the fact that deviations were approximately equally distributed in the positive and negative directions. In the LR direction, the standard deviation never exceeded 1 mm; however, the standard deviation was up to 1.5 mm in the AP direction. In the SI direction, the prostate was perceived to drift inferiorly (Figures 2 and 3) and the mean displacement was ≥0.5 mm for techniques with longer treatment session duration (IMATcc and IMRTcc), with a standard deviation exceeding 1.5 mm (for IMATcc and IMRTcc), see Table 2. To further evaluate the effect of time on intrafraction motion, tracking session motion of 1 mm amplitude increments were evaluated over 1-minute intervals (up to the mean tracking time for each corresponding treatment category). When plotted sequentially (Figure 4), an unambiguous trend of increasing motion with time was evident. Table 2 summarizes the results of the observed motion differences between the different treatment categories, indicating that geometric errors increase with increasing tracking time, with the greatest discrepancy observed in the SI followed by the AP direction.
Discussion
Multiple studies [13, 14] have shown that for treatment session time frames shorter than that for image-guided IMRT techniques, intrafraction displacement of the prostate position is insignificant. However, cine magnetic resonance imaging studies [15, 16] have shown significant prostate motion at time periods larger than 7 to 9.5 minutes, and have validated the correlation between prostate motion and rectal distension [12, 17]. In recent years, high sampling rate (that is, 10–30 Hz) fluoroscopic and electromagnetic systems have been introduced to facilitate on-line setup correction and continuous positional monitoring of implanted markers within target volumes. Consistent with the results of Shelton et al. [6], the current study presents an assessment of the temporal dependence of intrafraction motion on the prolongation of treatment time with real-time tumor tracking of electromagnetic transponders. Furthermore, the current study evaluated the impact of the addition of volumetric image-guidance onto IMRT techniques with the use of real-time localization and tracking. The major findings in the current study demonstrate that treatment time reduction for radiotherapy techniques without volumetric image-guidance resulted in significant reductions in motion error; Σ <0.6 mm and σ <1.2 mm compared with Σ ≤0.8 mm and σ <1.6 mm. The current findings are consistent with recent results by Mutanga et al. [18] for mean treatments time <5 minutes vs. >11 minutes. The current findings are also generally consistent with prior reports by Kotte et al. [10] for short (5–7 minutes) treatment durations.
For each treatment technique evaluated in this study, target displacements progressively increased as a function of elapsed treatment session duration (Figure 2). These findings may be attributable to increased motion around the mean position of the prostate, either internally (due to bladder and/or rectal filling), or externally (due to patient shifts) or related to gradual pelvic floor musculature relaxation [6]. Langen et al. showed that mean 3D motion >3 mm progressively increased with treatment time from 2% (during the first minute) to greater than 25% (after 10 minutes) [5]. The latter findings are in concordance with trends observed by Curtis et al. [7] and Xie et al. [19] for patients with treatment times >5 minutes and with the use of high and low sampling rate motion assessment techniques. These findings are also consistent with results in the current study (see posterior and/or inferior prostate drifts in Table 1). Together, these results are crucial, because intrafraction motion significantly contributes to the systematic and random errors of radiotherapy dose delivery [20] and may result in overdosing to the bladder and/or rectum as well as underdosing to the target volume.
Using a contribution of 2.5 times the systematic error and 0.7 times the random error [20], one can demonstrate that, for the shorter treatment categories (IMRTc and IMATc), the required margins are 1.0–1.1 mm (LR), 2.0–2.1 mm (AP), and 2.0 mm (SI). While these margins are consistent with those reported by Curtis et al. [7], Kotte et al. [10], and Xie et al. [19], they are not in agreement with those reported by Langsenlehner et al. [21], in part due to undersampling of motion trajectory with use of very low sampling rate techniques for target motion interrogation in the latter study. Correspondingly, the required margins for the longer tracking treatment categories (IMRTcc and IMATcc) are 1.5–1.6 mm (LR), 2.5 mm (AP), and 2.8–2.9 mm (SI). These are less than the ideal margins proposed by Curtis et al. [7] with volumetric imaging every 4 minutes to account for intrafraction motion errors in the absence of positional correction measures based on real-time continuous tracking. It is important to note that the margins reported here are not meant to define an absolute treatment margin due to the fact that only observed target motion is accounted for. Nevertheless, it is evident that comparatively larger margins are required to provide 95% isodose coverage for 90% of the sessions for the longer treatment categories. These large margins can be substantially reduced by continuous monitoring, implementation of an action threshold to reduce the effects of large motions, and keeping treatment sessions short—in this case, shorter that 6 minutes.
Clinical implications
Although the current results exhibit small intrafraction motion over entire treatment sessions, they also show that even with very accurate online position correction at the start of a treatment session, some positioning uncertainty remains and should be accounted for in margins. Aznar et al. [22] reported that when IMAT was used to treat prostate cancer patients, it required less than 2 minutes of beam-on time per treatment. In the current study, within 2 minutes after initial patient setup for daily treatment, the movement of the prostate was limited, though not trivial. Results in the current study suggest treatment session duration should not exceed 4–6 minutes. For treatment duration ≥4-6 minutes, and with the absence of real-time corrective measures for intrafraction motion, it is prudent to incorporate repeat patient localization [7]. Furthermore, in the absence of volumetric image-guidance for repeat patient localization, acquisition of at least the lateral component of an orthogonal image pair needs to be performed, since AP and SI directions are more susceptible to the temporal dependence of intrafraction motion.
Additional clinical strategies to stabilize the prostate gland with the goal of minimizing intrafraction motion include a controlled diet [23] and rectum filling [24–27]. However, these strategies have not garnered widespread clinical application, as their effectiveness when compared with faster treatment techniques, such as IMAT, is yet to be determined.
Limitations
Table adjustments were made for sustained prostatic displacements that exceeded a predetermined 4 mm threshold. Treatment interventions that occurred during tracking sessions were not retrospectively corrected to void the interventions. As such, the current analysis has the potential to underestimate displacements, particularly those >4 mm. The interpretation of the probability of displacement results, while easily understood from a geometric perspective, may be difficult to implement from a margin formation standpoint, hence, the application of this information to the generation of margins in radiation therapy may require additional evaluation.
Conclusion
Significant reductions in margins can be achieved through continuous tracking and intrafraction patient repositioning by means of threshold-based intervention. Prolonging treatment duration increases the likelihood of internal organ motion as well as external movement due to patient discomfort, which can impact overall treatment accuracy. Adopting treatment protocols such as IMAT combined with electromagnetic transponder detection and positioning, in addition to continuous monitoring of patient motion, provides an efficient, effective, and accurate delivery of external beam treatments. For treatment durations greater than 4–6 minutes and with no intrafraction motion mitigation protocol in place, patient repositioning is recommended, with the acquisition of at least the lateral component of an orthogonal image pair in the absence of volumetric imaging.
Abbreviations
- σ:
-
Random error
- Σ:
-
Systematic error
- AP/Y:
-
Anterior-posterior or frontal or vertical
- CT:
-
Computed tomography
- CBCT:
-
Cone-bean computed tomography
- Gy:
-
Gray
- IMAT:
-
Intensity-modulated arc therapy
- IMATc:
-
IMAT sessions with setup based on electromagnetic transponders only
- IMATcc:
-
IMAT sessions with setup based on electromagnetic transponders plus CBCT verification
- IMRT:
-
Intensity-modulated radiotherapy
- IMRTc:
-
IMRT sessions with setup based on electromagnetic transponders only
- IMRTcc:
-
IMRT sessions with setup based on electromagnetic transponders plus CBCT verification
- IRQ:
-
Interquartile range
- LR/X:
-
Lateral or left-right
- M:
-
Population-based mean
- RMS:
-
Root-mean-square
- SI/Z:
-
Longitudinal or cranio-caudal or superior-inferior
- std:
-
Standard deviation.
References
Dolezel M, Odrazka K, Vaculikova M, Vanasek J, Sefrova J, Paluska P, Zouhar M, Jansa J, Macingova Z, Jarosova L, Brodak M, Moravek P, Hartmann I: Dose escalation in prostate radiotherapy up to 82 Gy using simultaneous integrated boost: direct comparison of acute and late toxicity with 3D-CRT 74 Gy and IMRT 78 Gy. Strahlenther Onkol. 2010, 186: 197-202. 10.1007/s00066-010-2065-x.
Zelefsky MJ, Chan H, Hunt M, Schechter M, Kollmeier M, Cox B, Yamada Y, Fidaleo A, Sperling D, Happersett L, Zhang Z: Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006, 176: 1415-1419. 10.1016/j.juro.2006.06.002.
Dawson LA, Jaffray DA: Advances in image-guided radiation therapy. J Clin Oncol. 2007, 25: 938-946. 10.1200/JCO.2006.09.9515.
Krupa A, Fichtinger G, Hager GD: Real-time tissue tracking with B-mode ultrasound using speckle and visual servoing. Med Image Comput Comput Assist Interv. 2007, 10: 1-8.
Langen KM, Willoughby TR, Meeks SL, Santhanam A, Cunningham A, Levine L, Kupelian PA: Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008, 71: 1084-1090. 10.1016/j.ijrobp.2007.11.054.
Shelton J, Rossi PJ, Chen H, Liu Y, Master VA, Jani AB: Observations on prostate intrafraction motion and the effect of reduced treatment time using volumetric modulated arc therapy. Pract Radiat Oncol. 2011, 1: 243-250. 10.1016/j.prro.2011.02.008.
Curtis W, Khan M, Magnelli A, Stephans K, Tendulkar R, Xia P: Relationship of imaging frequency and planning margin to accoun for intrafraction prostate motion: Analysis based on real-time monitoring data. Int J Radiat Oncol Biol Phys. 2013, 85: 700-706. 10.1016/j.ijrobp.2012.05.044.
Poulsen PR, Cho B, Langen K, Kupelian P, Keall PJ: Three-dimensional prostate position estimation with a single x-ray imager utilizing the spatial probability density. Phys Med Biol. 2008, 53: 4331-4353. 10.1088/0031-9155/53/16/008.
Kitamura K, Shirato H, Seppenwoolde Y, Onimaru R, Oda M, Fujita K, Shimizu S, Shinohara N, Harabayashi T, Miyasaka K: Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int J Radiat Oncol Biol Phys. 2002, 53: 1117-1123. 10.1016/S0360-3016(02)02882-1.
Kotte AN, Hofman P, Lagendijk JJ, van Vulpen M, van der Heide UA: Intrafraction motion of the prostate during external-beam radiation therapy: analysis of 427 patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2007, 69: 419-425. 10.1016/j.ijrobp.2007.03.029.
Ghilezan M, Jaffray D, Siewerdsen J, Van Herk M, Shetty A, Sharpe MB, Zafar Jafri S, Vicini FA, Matter RC, Brabbins DS, Martinez AA: Prostate gland motion assessed with cine magnetic resonance imaging (cine-MRI). Int J Radiat Oncol Biol Phys. 2005, 62: 406-417. 10.1016/j.ijrobp.2003.10.017.
Tanyi JA, He T, Summers PA, Mburu RG, Kato CM, Rhodes SM, Hung AY, Fuss M: Assessment of planning target volume margins for intensity-modulated radiotherapy of the prostate gland: role of daily inter- and intrafraction motion. Int J Radiat Oncol Biol Phys. 2010, 78: 1579-1585. 10.1016/j.ijrobp.2010.02.001.
Wen N, Guan H, Hammoud R, Pradhan D, Nurushev T, Li S, Movsas B: Dose delivered from Varian’s CBCT to patients receiving IMRT for prostate cancer. Phys Med Biol. 2007, 52: 2267-2276. 10.1088/0031-9155/52/8/015.
Schallenkamp JM, Herman MG, Kruse JJ, Pisansky TM: Prostate position relative to pelvic bony anatomy based on intraprostatic gold markers and electronic portal imaging. Int J Radiat Oncol Biol Phys. 2005, 63: 800-811. 10.1016/j.ijrobp.2005.02.022.
Mah D, Freedman G, Milestone B, Hanlon A, Palacio E, Richardson T, Movsas B, Mitra R, Horwitz E, Hanks GE: Measurement of intrafractional prostate motion using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002, 54: 568-575. 10.1016/S0360-3016(02)03008-0.
Nichol AM, Brock KK, Lockwood GA, Moseley DJ, Rosewall T, Warde PR, Catton CN, Jaffray DA: A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys. 2007, 67: 48-56. 10.1016/j.ijrobp.2006.08.021.
Padhani A, Khoo V, Suckling J, Husband JE, Leach MO, Dearnaley DP: Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999, 44: 525-533. 10.1016/S0360-3016(99)00040-1.
Mutanga TF, de Boer HC, Rajan V, Dirkx ML, Incrocci L, Heijmen BJ: Day-to-day reproducibility of prostate intrafraction motion assessed by multiple kV and MV imaging of implanted markers during treatment. Int J Radiat Oncol Biol Phys. 2012, 83: 400-407. 10.1016/j.ijrobp.2011.05.049.
Xie Y, Djajaputra D, King CR, Hossain S, Ma L, Xing L: Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2008, 72: 236-246. 10.1016/j.ijrobp.2008.04.051.
van Herk M, Remeijer P, Rasch C, Lebesque JV: The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000, 47: 1121-1135. 10.1016/S0360-3016(00)00518-6.
Langsenlehner T, Döller C, Winkler P, Gallé G, Kapp KS: Impact of inter- and intrafraction deviations and residual set-up errors on PTV margins. Different alignment techniques in 3D conformal prostate cancer radiotherapy. Strahlenther Onkol. 2013, 189: 321-328. 10.1007/s00066-012-0303-0.
Aznar MC, Petersen PM, Logadottir A, Lindberg H, Korreman SS, Kjær-Kristoffersen F, Engelholm SA: Rotational radiotherapy for prostate cancer in clinical practice. Radiother Oncol. 2010, 97: 480-484. 10.1016/j.radonc.2010.09.014.
Lips IM, Kotte AN, van Gils CH, van Leerdam ME, van der Heide UA, van Vulpen M: Influence of antiflatulent dietary advice on intrafraction motion for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011, 81: e401-e406. 10.1016/j.ijrobp.2011.04.062.
Su Z, Zhao T, Li Z, Hoppe B, Henderson R, Mendenhall W, Nichols RC, Marcus R, Mendenhall N: Reduction of prostate intrafraction motion using gas-release rectal balloons. Med Phys. 2012, 39: 5869-5873. 10.1118/1.4749932.
Wang KK, Vapiwala N, Deville C, Plastaras JP, Scheuermann R, Lin H, Bar Ad V, Tochner Z, Both S: A study to quantify the effectiveness of daily endorectal balloon for prostate intrafraction motion management. Int J Radiat Oncol Biol Phys. 2012, 83: 1055-1063. 10.1016/j.ijrobp.2011.07.038.
Smeenk RJ, Louwe RJ, Langen KM, Shah AP, Kupelian PA, van Lin EN, Kaanders JH: An endorectal balloon reduces intrafraction prostate motion during radiotherapy. Int J Radiat Oncol Biol Phys. 2012, 83: 661-669. 10.1016/j.ijrobp.2012.01.080.
Both S, Wang KK, Plastaras JP, Deville C, Bar Ad V, Tochner Z, Vapiwala N: Real-time study of prostate intrafraction motion during external beam radiotherapy with daily endorectal balloon. Int J Radiat Oncol Biol Phys. 2011, 81: 1302-1309. 10.1016/j.ijrobp.2010.08.052.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1756-6649/13/4/prepub
Acknowledgement
This work was supported in part by funds from the Ted R. Lilly Cancer Continuing Umbrella of Research Education (CURE) Project (laureates: Mr. Alvaro Flores and Mr. Amanu G. Haile) and the Upward Bound Program at Portland State University, Portland, OR (laureates: Ms. Clarizza Catabay and Ms. Julia Nguyen). The authors are grateful to Ms. Clarizza Catabay, Ms. Julia Nguyen and Mr. Alvaro Flores for their independent contribution to study integrity validation. The authors are also grateful to Dr. Katelyn M. Atkins and Mr. Brandon Merz for providing careful review of the manuscript. Finally, the authors would like to acknowledge the professionalism, expertise and dedication of the radiation therapists at the Oregon Health & Science Knight Cancer Institute with special thanks to Dorothy J. Hargrove, M.S., R.T.(R)(T), Janet Garrett, R.T.(T), Robert Breckenridge, R.T.(T), Jennifer McLaughlin, R.T.(T), and Bonnie Luedloff, R.T.(T).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AC participated in the conception and design of the study, performed data analysis, evaluated the results, and drafted the manuscript. AGH participated in the conception and design of the study, performed data analysis, evaluated the results, and drafted the manuscript. SO participated in the conception and design of the study, performed data analysis, evaluated the results, and drafted the manuscript. TSD participated in the design of the study, performed data analysis, evaluated the results, and drafted the manuscript. WMR participated in the design of the study, performed data analysis, evaluated the results, and drafted the manuscript. KER participated in the design of the study, and revised the manuscript. NN participated in the design of the study, and revised the manuscript. TN participated in the statistical analytical assessment of the study data. LZM was responsible for data acquisition, data analysis, evaluation of results, and revision of the manuscript. MF participated in the design of the study, and revised the manuscript. JAT participated in the conception and design of the study, performed data analysis, evaluated the results, and drafted and revised the manuscript. AYH treated all the patients that formed the basis of this study, participated in the design of the study and data analysis, and revised the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Cramer, A.K., Haile, A.G., Ognjenovic, S. et al. Real-time prostate motion assessment: image-guidance and the temporal dependence of intra-fraction motion. BMC Med Phys 13, 4 (2013). https://doi.org/10.1186/1756-6649-13-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-6649-13-4