Abstract
Background
Members of the Anopheles gambiae complex are amongst the best malaria vectors in the world, but their vectorial capacities vary between species and populations. A large-scale sampling of An. gambiae sensu lato was carried out in various bioclimatic domains of Madagascar. Local abundance of an unexpected member of this complex raised questions regarding its role in malaria transmission.
Methods
Sampling took place at 38 sites and 2,067 females were collected. Species assessment was performed using a PCR targeting a sequence in the IGS of the rDNA. Analysis focused on the relative prevalence of the species per site, bioclimatic domain and altitude. Infectivity of Anopheles merus was assessed using an ELISA to detect the presence of malarial circumsporozoite protein in the head-thorax.
Results
Three species were identified: An. gambiae, Anopheles arabiensis and An. merus. The distribution of each species is mainly a function of bioclimatic domains and, to a lesser extent, altitude. An. arabiensis is present in all bioclimatic domains with highest prevalence in sub-humid, dry and sub-arid domains. An. gambiae has its highest prevalence in the humid domain, is in the minority in dry areas, rare in sub-humid and absent in sub-arid domains. An. merus is restricted to the coastal fringe in the south and west; it was in the majority in one southern village. The majority of sites were sympatric for at least two of the species (21/38) and two sites harboured all three species.
The role of An. merus as malaria vector was confirmed in the case of two human-biting females, which were ELISA-positive for Plasmodium falciparum.
Conclusion
Despite the huge environmental (mainly man-made) changes in Madagascar, the distribution of An. gambiae and An. arabiensis appears unchanged for the past 35 years. The distribution of An. merus is wider than was previously known, and its effectiveness as a malaria vector has been shown for the first time; this species is now on the list of Malagasy malaria vectors.
Similar content being viewed by others
Background
The Anopheles gambiae complex plays a central role in malaria transmission. Numbers of studies have been conducted to draw the picture of its distribution in Africa south of the Sahara, Madagascar and small, related islands. Distribution maps have been made based on thousands of observations [1–3].
Madagascar is an area of special interest for such studies because it concentrates in a relatively small area most of the habitats colonised by members of this complex. In the available literature on the distribution of An. gambiae complex in Madagascar three contributions are prominent.
-
Grjebine [4] studied the relative abundance of An. gambiae sensu lato as compared to other anopheline species, without making any distinction between the "freshwater species A and B". He demonstrated a continuous distribution at altitude < 1400 m. No saltwater species was identified but Anopheles merus was raised from larvae found in crab-holes in the Betsiboka estuary (near Mahajanga) with 0.7 g NaCl / l.
-
Chauvet [5] distinguished the two freshwater species An. gambiae A (An. gambiae sensu stricto) and An. gambiae B (Anopheles arabiensis). Specific determination was performed in London using cross-mating protocols with reference strains, a method judged incontestable [6]; it was also performed in Madagascar using morphometric examination of the mesothoracic seta No.1 of the 4th larval stage, a method judged acceptable if at least 25 larvae are examined from the same progeny [7, 8]. Overall, 1,481 specific identifications were performed from females collected between 1964 and 1969 in 133 sites distributed in the whole Madagascar. An. gambiae represented 46% and An. arabiensis 54% of the total. 47% of sites were sympatric for these two species. An. arabiensis was observed everywhere and was the main species in western and highland regions. An. gambiae was absent from the southern region and was the main species in the eastern region. The presence of An. merus was established in only one place, a salt marsh near Toliara (=Tuléar), using the cross-mating method, with a solution of 18.4 g NaCl / l as the breeding water, and using a physiological survival test which involved placing first instar larvae in a solution of 75% sea water for 6 hours.
-
Ralisoa [9] separated species of the An. gambiae complex using differences in the banding patterns of polytene chromosomes in the ovarian nurse cells of half gravid females [10]. Overall 25 sites were visited and 3,625 females were examined. A large amount of information was obtained on chromosomal inversions. Distribution and relative abundance of An. gambiae and An. arabiensis were strictly concordant with Chauvet's findings.
Numerous methods are now available to enable the distinction of various species of this complex. Apart from the methods previously mentioned (cross-mating, cytogenetics), isoenzymatic analysis [11], cuticular hydrocarbon analysis [12] and the use of DNA probes [13] present some interest. The advent of molecular biology tools, especially the PCR multiplex technique, opens new avenues of research: this method enables easy determination of sex, stage and physiological status using only a fragment of a mosquito morphologically assigned to An. gambiae s.l. [14]. This method was used to update the distribution of the An. gambiae in Madagascar and to determine the ecological factors that influence the observed distribution of each species on the basis of bioclimatic domains and altitudes.
Materials and Methods
Five bioclimatic domains and their limits have been defined by Cornet [15], briefly as follows:
-
the humid domain on the east coast, where precipitation is higher than 2,000 mm and observed throughout the year, together with high mean temperatures and hygrometry;
-
the sub-humid domain includes the highland areas and Sambirano (in the north-west) where precipitation ranges between 800 and 1500 mm with a dry season of 5–6 months;
-
the mountain domain at altitudes > 2000 m, where precipitation is above 2,000 mm and observed throughout the year; this domain is least common and constitutes only 0.35% of the surface area of Madagascar; furthermore the presence of An. gambiae s.l. has never been documented or suspected (for which is why this domain is not mentioned in the results of this study);
-
the dry domain in the west and north where precipitation ranges between 800 and 1500 mm with a dry season > 7 months;
-
and the sub-arid domain in the south, comparable to the Sahel in mainland Africa, where precipitation ranges between 300 and 600 mm with rains for only two months.
The sampling of mosquitoes was performed between March 1996 and March 2003 by various means: human landing collection outdoors and indoors, pyrethrum spray catches, Muirhead-Thomson's artificial pit shelters [16], and larval collections. Overall, 38 sites distributed throughout Madagascar and belonging to 4 bioclimatic domains were investigated. Most of the collections were unique events but few of them came from longitudinal survey constituted by repeated observations with a monthly periodicity. Specimens of female mosquitoes were preserved at +4°C, dry in silica gel or in 95% ethanol. The Ministry of Health of Madagascar approved the protocol. While the used method of mosquitoes favoured the collection of An. gambiae s.l., other mosquitoes among which number of anophelines were obtained; one of these, namely An. funestus, is a major malaria vector throughout Madagascar and will be considered in other forthcoming paper. Water quality measurements for Na+, K+, Ca++ and Mg++ of larval breeding places were performed with emitting photometry using a flame photometer Ciba-Corning 400 without internal calibration.
Mosquito DNA was obtained in two ways: 1) after crushing one leg, the DNA was extracted [17] then resuspended in distilled water 100 μl, or 2) one leg was put directly in the reaction master mix [18]. 25 μl of this latter were composed of buffer 10X (100 mM tris pH 8.3, KCl 500 mM, MgCl2 15 mM, 0.1% (w/v) gelatin) 2.5 μl, dNTP (solution at 10 mM for each nucleotide) 0.5 μl, primers (20 ng/μl) 1 μl, Taq polymerase (Amersham™) 1 unit, DNA extract 5 μl (or whole leg) and sterile distilled water 13.8 μl. Five primers of 20 nucleotides were used, of which 4 were specific to An. gambiae, An. arabiensis, An. merus, Anopheles quadriannulatus and one was common to all species of the An. gambiae complex [19]. These primers distinguish specific differences in IGS of rDNA in the heterochromatin of the X chromosome. Amplification has been performed with the following programme : initial denaturation at 94°C for 5 mn, 30 cycles with denaturation at 94°C for 30 s, annealing at 55°C for 30 s and elongation at 72°C for 1 mn, and final elongation at 72°C for 10 mn. Amplification fragments were separated by electrophoresis in an agarose gel (1.5%) containing ethidium bromide revealing PCR products of 153 pb for An. quadriannulatus B, 315 pb for An. arabiensis, 390 pb for An. gambiae, 466 pb for An. merus. Mosquitoes that remained "PCR negative" (i.e. without any band on the gel) were all tested three times, in independent manners. Negative controls have been systematically realised during PCR and electrophoresis using DNA negative mix.
Results
After a first PCR on 2,067 females of the An. gambiae complex, 397 mosquitoes were "PCR negative", and the number decreased to 75, then 45, after a second and a third PCR runs.
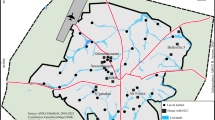
Mosquitoes (Tables 1 and additional file 1) were collected at 38 sites distributed within 4 bioclimatic domains (Figure 1 and additional file 1). The number of mosquitoes per site averaged 54.4 and ranged between 7 and 184. 2,022 Two thousand twenty two mosquitoes (98%) were assigned to one of the 3 species: An. gambiae, An. arabiensis and An. merus that represented 37%, 59% and 4% of the determined mosquitoes, respectively. No hybrids were observed. Most of the sampled sites (21/38 = 55%) were sympatric areas for ≥ 2 species. Two sites harboured all 3 species (No.16 Ankiliefatra and No.38 Mangatsa). An. merus was always observed in sympatry with An. arabiensis.
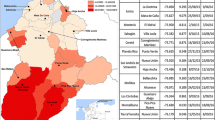
Distribution of the species of the Anopheles gambiae complex in Madagascar. Bioclimatic domains, their definitions and limits are as described by Cornet 1974. The names of precise localisation of the 38 sampled sites are in additional file 1. Black squares represent the 6 main towns with, from the North to South, Antsiranana, Mahajanga, Toamasina, Antananarivo, Fianarantsoa and Toliara.
An. gambiae represented 93% of the species in the humid domain. It was more common in dry than in sub-humid domains, and more frequently found in the latter than in sub-arid domains. With the exception of a unique specimen (at site No.16 Ankiliefatra), it was absent from the sub-arid domain. It was present in the sub-humid domain at altitude ≤ 900 (site No.33 Saharevo, at 900 m and site N°36 Manjakavaradrano at 800 m). Altitude was clearly a limiting factor: at ≥ 700 m this species represented 4% of the determinations and was absent at ≥ 1000 m with the exception of 2 specimens (site No.32 Ankazobe, altitude 1.300 m).
An. arabiensis was prevalent in sub-humid, sub-arid and dry domains. It was rare in humid domain but observed at most sites (10/13). Altitudes of up to 1,515 m (in site No.26 Tsarazaza) did not limit its distribution. The ratio gambiae/gambiae+arabiensis was tested with indoor- (41.2%) and outdoor-landing collections (35.6%); the difference was significant (p = 0.03 by Fisher's exact test).
An. merus presented a restricted distribution area. It was observed only in the extreme south, in the driest part of the sub-arid domain and in one site (No.38 Mangatsa) in the dry domain. It was always observed at ≤ 20 km from the seashore. It was observed to be the main species at one site (69% of identified mosquitoes in March 2003 at site No.16 Ankiliefatra). It was collected at the adult stage using various methods (indoor and outdoor human landing, indoor resting, indoor and outdoor miniature light-trap) and at the larval stage in water that was analyzed (in mM/l) as follows: Na+ 13, K+ 0.3, Ca++ 0.62, Mg++ 1.1. An. merus did not seem to depend on mangrove swamps, since there are no mangrove in the extreme south, or near site No.38 (Mangatsa) where it has been observed; furthermore, a large mangrove exists in one site (No.28 Kimony) but no An. merus was observed there.
During the whole longitudinal survey, performed in 1995 in a village of the sub-arid domain (site No.16 Ankiliefatra) 275 An. gambiae s.l. collected by human landing were tested by ELISA [20] to detect the presence of circumsporozoite protein in the head-thorax. Two females, one indoor in January and one outdoor in August, were positive for P. falciparum; both were An. merus. The unique An. merus collected by indoor-resting near Mahajanga (in site No.38 Mangatsa) was freshly-fed with human blood.
Discussion
The relative prevalence and distribution limits of the two main species An. gambiae and An. arabiensis have remained extremely stable since the first available data [5], more than 35 years ago. Attentive examination did not reveal notable differences. However, huge environmental modifications have occurred, mainly due to demographic increase and human activity resulting in dramatic deforestation and an increase in the areas given over to rice-fields. This did not obviously affect the distribution of the members of the An. gambiae complex.
Because An. arabiensis is present in all regions, the question of understanding the limiting factors of distribution is only of concern in the case of An. gambiae. Bioclimatic domain and altitude appear to be two key variables. An. gambiae is limited in its habitat in Madagascar by only two factors: aridity in the south and south-west and altitude > 1000 m. This picture is perfectly concordant with the findings of this study, except at site No.1 Ampasimajina), perhaps due to the small sample size, near a place in which Chauvet [5] had identified 3 An. gambiae s.l. in December 1966. The moisture that favours An. gambiae may explain why some individuals have been observed at low prevalence (always <5%) during the period of maximum rainfall in the northern and western margins of the central highlands (site N°32 Ankazobe, and also N°25 Fenoarivo with 1 An. gambiae caught in March 1997). A recent study performed in Kenya [21] presents two interesting findings: as in Madagascar the moisture index was the most important variable explaining the distribution of An. gambiae, but unlike Madagascar An. arabiensis was not observed in areas above 1400 m in western Kenya.
An. merus was collected from 3 different areas: in the extreme south and a site near Mahajanga in these studies and had been previously collected by Chauvet in a salt swamp near Toliara [5]. It is possible that all the western and southern coasts have suitable habitats but the observation that An. merus is not closely associated with mangroves (in line with Chauvet's findings [5]) does not support this assumption. Why is this species not observed on the west coast? This is surprising in view of the climatic conditions that prevail, which resemble those observed on the east coast of Kenya where An. merus is found [2]. The different nature of the soils between west and east coast in Madagascar may explain this: mainly sedimentary rocks (alluvial and lake deposits, unconsolidated sands) in the west and metamorphic rocks (basement rocks, lava) in the east [22].
It is likely that the collection methods influenced the observed distributions, but this influence is not considered to lead to a major bias. The ratio gambiae/gambiae+arabiensis indoor- versus outdoor-landing collections was significantly different, with a higher exophily for An. arabiensis as classically observed in continental Africa, but the percentages were rather similar (41.2% indoor and 35.6% outdoor). Furthermore, each of the 3 species was collected using indoor-landing, outdoor-landing and pyrethrum spray collections. Considering the large variations in zoophilic behaviour of populations of the An. gambiae complex only tens of kilometres apart [23], an ideal sampling would involve at least 3 different collection methods in each prospected site, which was only achieved in 10 of the 38 sites sampled.
Another point that merits discussion is that of the 45 "PCR negative" mosquitoes. It is conceivable that PCR sometimes fails: this is supported by the decreasing number of "PCR negative" mosquitoes after second then third attempt. Poor DNA is also a possibility. The eventuality of a fourth and unknown species of the An. gambiae complex present in Madagascar, is an unlikely hypothesis but one which cannot be ruled out. Overall, the PCR multiplex method gave reliable results for 98% of tested mosquitoes, which is considered an acceptable result.
An. merus is confirmed as having a role as a malaria vector in Madagascar, which had not previously been recorded. These observations are compatible with those performed in Tanzania where, in places, it is the main vector [24]. A fifth species can now be added to the list of anopheline vectors of human malaria in Madagascar: An. gambiae, An. arabiensis, An. funestus, An. mascarensis and now An. merus.
Conclusions
Despite the huge environmental changes in Madagascar, the distribution of An. gambiae and An. arabiensis appear to have remained constant over the past 35 years. The distribution of An. merus is wider than was previously known and its effectiveness as a malaria vector has been recorded for the first time.
References
Gillies MT, De Meillon B: The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region). 1968, Johannesburg: The South African Institute for Medical Research, [publication n°54]
Gillies MT, Coetzee M: A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). 1987, Johannesburg: The South African Institute for Medical Research, [publication n°55]
Coetzee M, Graig M, Le Sueur D: Distribution of African malaria mosquitoes belonging to the Anopheles gambiae complex. Parasitol Today. 2000, 16: 74-77. 10.1016/S0169-4758(99)01563-X.
Grjebine A: Insectes Diptères Culicidae Anophelinae. In Faune de Madagascar. 1966, Paris: ORSTOM/CNRS, 22: 1-487.
Chauvet G: Répartition et écologie du complexe Anopheles gambiae à Madagascar. Cahiers ORSTOM Sér Ent Méd Parasitol. 1969, 7: 235-278.
Davidson G: Anopheles gambiae complex. Nature. 1962, 196: 907-
Chauvet G, Déjardin J: Caractères chétotaxiques de distinction entre larves (stade IV) de l'espèce A et de l'espèce B du complexe Anopheles gambiae à Madagascar. Cahiers ORSTOM Sér Ent Méd Parasitol. 1968, 6: 69-101.
Chauvet G, Davidson G, Déjardin J: Validité d'une méthode chétotaxique de distinction des larves des espèces A et B du complexe Anopheles gambiae Giles à Madagscar. Cahiers ORSTOM Ser Ent Med Parasitol. 1969, 7: 51-60.
Ralisoa OB: Biogéographie du complexe Anopheles gambiae de Madagascar, vecteur du paludisme. In Biogéographie de Madagascar. Edited by: Lourenço WR. 1996, Paris: ORSTOM Editions, 523-534. [Collection Colloques et Séminaires]
Coluzzi M, Sabatini A, Petrarca V, Di Deco MA: Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979, 73: 483-497.
Miles SJ: A biochemical key to adult members of the Anopheles gambiae group of species (Diptera : Culicidae). J Med Entomol. 1979, 15: 297-299.
Carlson DA, Service MV: Identification of mosquitoes of the Anopheles gambiae species complex A and B by analysis of cuticular components. Science. 1980, 207: 1089-1091.
Gale KR, Crampton MC: DNA probes for species identification of mosquitoes in the Anopheles gambiae complex. Med Vet Entomol. 1987, 1: 127-136.
Paskewitz SM, Collins FH: Use of the polymerase chain reaction to identify mosquito species of the Anopheles gambiae complex. Med Vet Entomol. 1990, 4: 367-373.
Cornet A: Essai de cartographie bioclimatique à Madagascar, carte à 1/2 000 000 et notice. 1974, Paris: Editions ORSTOM, [http://www.mobot.org/MOBOT/research/madagascar/maps/bc5tif.jpg]
Muirhead-Thomson RC: A pit shelter for sampling outdoor mosquito populations. Bull World Health Organ. 1958, 19: 1116-1118.
Cornel AJ, Coetzee M, Van Rensburg AJ, Koekemoer LL, Hunt RH, Collins FH: Ribosomal DNA-polymerase chain reaction assay discriminates between Anopheles quadriannulatus and An. merus (Diptera: Culicidae). J Med Entomol. 1997, 34: 573-577.
Fontenille D, Faye O, Konaté L, Sy N, Collins FH: Comparaison des techniques PCR et cytogénétique pour la détermination des membres du complexe Anopheles gambiae au Sénégal. Ann Parasitol Hum Comp. 1993, 68: 239-240.
Scott JA, Brogdon WG, Collins FH: Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993, 49: 520-529.
Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG: Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull WHO. 1987, 65: 39-45.
Minakawa N, Sonye G, Mogi M, Githeko A, Yan G: The effect of climatic factor on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002, 39: 833-841.
Madagascar simplified database. [http://149.170.199.144/new_gis/madagas/gisdata/geolslar.gif]
Duchemin JB, Léong Pock Tsy JM, Rabarison P, Roux J, Coluzzi M, Costantini C: Zoophily of Anopheles arabiensis and An. gambiae in Madagascar demonstrated by odour-baited entry traps. Med Vet Entomol. 2001, 15: 50-57. 10.1046/j.1365-2915.2001.00276.x.
Temu EA, Minjas JN, Coetzee M, Hunt RH, Shift CJ: The role of four anopheline species (Diptera: Culicidae) in malaria transmission in coastal Tanzania. Trans R Soc Trop Med Hyg. 1998, 92: 152-158.
Acknowledgements
The help of the technical team of the Medical Entomology Laboratory of the Institut Pasteur de Madagascar is greatly appreciated. Dr Remi Laganier is thanked for performing water quality measurements. Funds were provided by the Programmes VIH/PAL and PAL+ ("Anopheles d'Afrique" network) from the French Ministry of Research, the Project FAC-94006000 from the French Ministry of Cooperation, the Institut Pasteur de Madagascar and IRD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
JMLPT provided the data sheet and drafted the manuscript. JBD conceived the study. PR undertook the technical work. ViRo analysed the results and drafted the manuscript. All authors participated in field sampling, contributed to the writing of the manuscript and approved the submitted version of the manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Pock Tsy, JM.L., Duchemin, JB., Marrama, L. et al. Distribution of the species of the Anopheles gambiae complex and first evidence of Anopheles merus as a malaria vector in Madagascar. Malar J 2, 33 (2003). https://doi.org/10.1186/1475-2875-2-33
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-2-33