Abstract
Background
The optimal timing of pulmonary homograft valve replacement (PVR) is uncertain. Cardiopulmonary exercise testing (CPET) and cardiac magnetic resonance (CMR) are often used to guide the clinical decision for PVR in operated tetralogy of Fallot (TOF) patients with significant pulmonary regurgitation (PR). We aim to study the relationship between exercise capacity and CMR in these patients.
Methods
The study is a single-centre retrospective analysis of 36 operated TOF patients [median 21.4 (interquartile range 16.4, 26.4) years post-repair; 30 NYHA I, 6 NYHA II; median age 25.2 (interquartile range 19.5-31.7) years, 29 males] with significant PR on CMR who underwent CPET within 15 [median 2.0 (interquartile range 0.8-7.2)] months from CMR. CPET parameters were compared with 30 age- and sex-matched healthy controls [median age 27.8 (interquartile range 21.0-32.8) years; 24 males].
Results
Peak systolic blood pressure (177 versus 192 mmHg, p = 0.007), Mets (7.3 versus 9.9, p < 0.001), peak oxygen consumption (VO2max) (29.2 versus 34.5 ml/kg/min, p < 0.001) and peak oxygen pulse (11.0 versus 13.7 ml/beat, p = 0.003) were significantly lower in TOF group versus control. Univariate analyses showed negative correlation between PR fraction and anaerobic threshold. There was a positive correlation between indexed left (LV) and right (RV) ventricular end-diastolic volumes, as well as indexed LV and effective RV stroke volumes, on CMR and VO2max and Mets achieved on CPET. These remained significant after adjustment for age and sex.
Conclusions
TOF subjects have near normal exercise capacity but significantly lower Mets, VO2max and peak oygen pulse achieved compared to controls. Increased PR fraction in TOF subjects was associated with lower anaerobic threshold. Higher indexed effective RV stroke volume, a measure of LV preload, was associated with higher VO2max and Mets achieved, and may potentially be used as a predictor of exercise capacity.
Similar content being viewed by others
Background
Long-term outcomes of patients with tetralogy of Fallot (TOF) have improved with advances in surgical techniques [1]. However, pulmonary regurgitation (PR) resulting from repair to the right ventricular outflow tract is a common consequence of the primary surgery. This is usually well tolerated initially, but leads to increased right ventricular (RV) dilatation, dysfunction and worsening exercise intolerance over time, and may result in arrhythmia and sudden cardiac death [2–4].
The optimal timing of pulmonary homograft valve replacement (PVR) in operated TOF patients with significant PR is unclear. The decision for PVR is usually a fine balance between avoiding permanent remodeling of the right ventricle [5, 6] and reducing the need for repeat procedures throughout one’s lifetime in these often young patients. Cardiopulmonary exercise testing (CPET) and cardiac magnetic resonance (CMR) are two important tools often used by clinicians to help guide this difficult management decision [5, 6].
Poor exercise capacity identifies patients at higher risk of hospitalization and mortality [7–9] and guidelines have listed decreasing exercise tolerance as a strong indication for PVR [10–12]. The American College of Cardiology/American Heart Association (ACC/AHA) 2008 guidelines recommend PVR as a Class I indication in operated TOF patients with severe PR and decreased exercise tolerance [12]; exercise testing is a useful adjunct. A Class IIa indication for PVR is in patients with severe PR and moderate to severe RV enlargement [12]. The exact definition of moderate to severe RV dysfunction/enlargement is not clear. The European Society of Cardiology (ESC) 2010 guidelines have similar recommendations but suggest using a RVEDVi cut-off of 160 ml/m2, beyond which normalization of RV becomes unlikely [10]. The Canadian Cardiovascular Society (CCS) 2009 guidelines recommend as a Class IIa indication that PVR should be considered in TOF patients with severe PR who have either a deterioriating exercise performance or moderate to severe RV enlargement (RVEDVi >170 ml/m2) [11]. A RVEDVi cut-off of 150 ml/m2 is sometimes used clinically [13]. A recent study found similar exercise capacity and stroke volumes in patients below and above the threshold, thus raising questions about the utility of RVEDVi as the main basis for deciding on timing for PVR [14].
The relationship between CMR parameters and actual exercise capacity remains unclear. Understanding the relationship between exercise capacity and CMR parameters is important and may better help rationalize the clinical decision for PVR. We aim to study the exercise capacity in TOF patients with significant PR late (>10 years) after repair and its relationship with CMR parameters.
Methods
Study design and patient population
In our institution, operated adult TOF patients with significant (at least moderate) PR on TTE will undergo CMR and CPET as part of their clinical management. The study is a retrospective review of all consecutive patients with surgically corrected TOF and known significant (moderate to severe) PR on TTE, who underwent CMR and CPET at our institution from Apr 2005 to Mar 2013. All patients were older than 15 years of age and had undergone corrective surgery for TOF more than 10 years previously [median 21.4 years (IQR 16.4, 26.4)]. Patients with residual pulmonary stenosis pressure gradient on Doppler echocardiography, associated atrioventricular septum defect, double outlet right ventricle of Fallot type, pulmonary atresia with ventricular septum defect, TOF with absent pulmonary valve, or patients who had undergone pulmonary valve replacement (PVR) were excluded. Clinical data were obtained from review of patients’ case-files and electronic medical records.
CMR and CPET data were recorded. CPET data were also obtained from age- and sex-matched healthy controls. The study was approved by the SingHealth Centralised Institutional Review Board (SingHealth, Singapore).
Cardiac MRI
Study subjects underwent CMR on a 1.5 T Avanto scanner (Siemens). In each patient, fast imaging in steady-state precession (trueFISP) cine MR images were acquired in the left ventricular (LV) long axes (2-, 3- and 4-chamber views), RV outflow tract (RVOT) (oblique sagittal and coronal) planes as well as a parallel contiguous stack of 8 mm ventricular short-axis cine slices. Retrospective ECG-triggering was employed, achieving typical frame rates of about 25 per cardiac cycle. Throughplane flow measurements in planes across the aortic root and main pulmonary trunk were performed using velocity-encoded gradient-echo pulse sequences.
Calculation of left and right ventricular end-diastolic (EDV) and end-systolic (ESV) volumes, LV mass and ejection fractions (EF) was performed using CMRTools (London, UK). All volumes and LV mass measurements were then indexed to body surface area. The presence of RVOT aneurysm, defined as akinesia or dyskinesia (outward movement) during systole of part of the RVOT assessed from the basal ventricular short-axis cine images, was recorded. PR fraction (diastolic reverse flow expressed as a percentage of forward flow) was assessed from anterograde and retrograde flow in the main pulmonary artery (ARGUS software, Siemens, Erlangen). Effective right ventricular stroke volume (RVSV) was calculated as the difference between the total RV forward flow and the pulmonary regurgitant flow [15, 16]. Restrictive RV physiology was ascertained by presence of end-diastolic forward pulmonary flow, consistent with severely limited RV capacitance during right atrial contraction.
Cardiopulmonary exercise testing
Cardiopulmonary exercise tests were performed on a Hewlett-Packard Cosmos treadmill machine. A maximal ramp protocol with fixed treadmill speed but constantly increasing incline gradient to provide an increasing workload was employed. Exercise speed was determined at the performing physician's discretion. Patients were encouraged to exercise until symptoms were intolerable, i.e. when Rating of Perceived Exertion reached 18/20. Heart rate was monitored continuously, and non-invasive blood pressure was taken every 2 minutes with both manual and automatic blood pressure. Oxygen saturation was monitored by a finger probe pulse oximeter. Oxygen uptake (VO2), minute ventilation (VE), and carbon dioxide output (VCO2) were measured breath by breath. The anaerobic threshold (AT) was chosen as the VO2 at which respiratory exchange ratio reached 1.0 and when there was a sudden and sustained precipitous rise in VCO2. Peak VO2 was defined as the value of averaged data during the final 15 seconds of exercise. The VE/VCO2 slope was determined as the linear regression slope of VE and VCO2.
Statistical analysis
Data was analysed using STATA version 11.1 (College Station, Texas, USA). All values were presented as median and interquartile range for continuous data and frequency and percentage for categorical data. Comparisons between groups were made with Mann–Whitney U test, or the Fisher’s exact test as appropriate. The linear correlation, r, between exercise testing and CMR parameters were initially assessed using Pearson correlation coefficient with 95% confidence interval (CI). Significant relationships were subsequently assessed by multiple linear regression technique adjusting for age and gender. Scatter plots with linear fit were produced. For all analyses, a two-tailed P value of <0.05 was considered significant.
Results
Baseline clinical characteristics
Of the 119 TOF patients on clinical follow-up at our institution at the beginning of the study, 36 had significant PR on echocardiography. The latter underwent CPET and CMR testing and were included in this study. Thirty controls were also recruited. There was no significant difference in age, sex and indexed body mass between patients and controls. Six TOF patients had NYHA II functional status; the rest of the study TOF population were either asymptomatic or NYHA I (Table 1).
CPET parameters
Compared to controls, TOF patients achieved significantly lower maximum systolic blood pressure (177 versus 192 mmHg, p = 0.007), Mets (7.3 versus 9.9, p < 0.001), peak oxygen consumption (29.2 versus 34.5 ml/kg/min, p < 0.001) and peak oxygen pulse pressure (11.0 versus 13.7 ml/beat, p = 0.003). These differences were significant even after adjusting for age and sex. There were no significant difference in the rest of the CPET parameters (Table 1). 7 TOF patients experienced arrhythmia during CPET, all consisting of occasional isolated premature ventricular complexes. No malignant arryhtmia was noted.
Medication, Surgical and CMR findings in TOF patients
The majority of the TOF patients were not on any cardiac medications: one patient was on beta-blocker and ACE inhibitor; one patient, ACE inhibitor and diuretics; and one patient, beta-blocker alone. The median age at which surgical repair was performed was 3.7 years (IQR 2.3-6.9) and the median time from surgical repair was 21.4 years (IQR 16.4-26.4). Ten patients (27.8%) had undergone transannular patch repair (Table 2). All patients were in sinus rhythm.
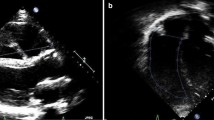
The median RVEDVi, RVESVi, RVEF, RVSVi, effective RVSVi and PR fraction were 167.7 ml/m2 (IQR 142.5-178.5), 87.2 ml/m2 (IQR 75.8-98.4), 45.6% (IQR 39.2-50.0), 73.5 ml/m2 (IQR 61.8-82.9), 41.1 ml/m2 (37.9-44.3) and 45.0% (IQR 35.5-52), respectively. The median LVEDVi, LVESVi, LVEF and LVSVi were 73.1 ml/m2 (IQR 66.6-82.5), 30.4 ml/m2 (IQR 26.9-35.3), 58.7% (IQR 52.9-62.3) and 43.1 ml/m2 (IQR 39.0-48.1), respectively. All patients had LVEF >40%, with five (13.9%) patients having LVEF between 40-50%. Twenty-six (72.2%) had restrictive RV physiology; and 20 (55.6%), RVOT aneurysms (Figure 1).
Relationship between CPET and CMR parameters in TOF patients
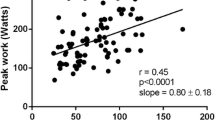
The median time between CPET and CMR was 2.0 months (IQR 0.8-7.2); there was no change in clinical status between CPET and CMR for all patients. Larger RVEDVi (r = 0.36, 95% CI 0.03-0.62, p = 0.035), RVESVi (r = 0.35, 95% CI 0.01-0.61, p = 0.042) and LVEDVi (r = 0.39, 95% CI 0.06-0.64, p = 0.021) were associated with significantly higher Mets achieved during exercise testing. Larger RVEDVi (r = 0.35, 95% CI 0.03-0.61, p = 0.037) and LVEDVi (r = 0.35, 95% CI 0.03-0.61, p = 0.030) were associated with higher peak oxygen consumption achieved during exercise testing. Larger LVSVi (r = 0.37, 95% CI 0.04-0.62, p = 0.030) and effective RVSVi (r = 0.53, 95% CI 0.24-0.73, p = 0.001) were associated with significantly higher Mets achieved. Larger LVSVi (r = 0.35, 95% CI 0.02-0.61, p = 0.037) and effective RVSVi (r = 0.53, 95% CI 0.24-0.73, p < 0.001) were associated with significantly higher peak oxygen consumption. Greater PR fraction was associated with lower AT (r = -0.40, 95% CI -0.65 to 0.07, p = 0.020)(see Table 3). After adjusting for age and sex, all these relationships remained significant (Table 4, Figure 2). Of note, there was no significant correlation between exercise data and either RVEF or RVSV. Comparing TOF patients with and without restrictive RV physiology, no significant difference in CPET parameters was observed. This was the same among those with and without RVOT aneurysms. There was also no significant association between the rest of the CMR parameters and CPET observations.
Discussion
Our operated TOF patients with significant PR had near normal exercise capacity, albeit lower than that of normal healthy controls. Although direct comparison is difficult because of differences in cohort characteristics, the exercise capacity in our operated TOF patients with significant PR is fairly similar to a published series [8] and better compared to others [7, 17]. These latter studies included patients with all degrees of PR, unlike our study in which only patients with significant PR on echocardiography were studied with CMR and CPET. In clinical practice, only patients with significant PR routinely undergo surveillance cardiac MR. Our study thus provides clinically-relevant data in the subset of patients with significant PR.
We demonstrated a negative correlation between PR fraction and AT, implying a possible deleterious effect on exercise capacity of worsening PR per se. The AT is decreased in patients with heart disease and is an important functional limit: physiological responses to exercise are different above and below the AT [18]. While low AT in heart failure patients is associated with increased mortality [19], the prognostic value of AT in TOF patients has not been previously studied.
In our study, we found that higher effective RV stroke volume correlated significantly with higher Mets and peak oxygen consumption. In contrast, we found no significant correlation between exercise capacity and either RVEF or RV stroke volume, which is in keeping with reported studies [14, 20]. In our TOF cohort, which has similar distribution of RV and LV volume and ejection fraction parameters to that of other studies [14, 21], the majority had normal LV function. Conceivably, the main determinant of cardiac output (and thereby exercise capacity) would thus be LV preload. In patients with significant PR, LV preload may best be assessed by effective RVSVi as LV preload is determined by the actual net RV forward flow, taking into account the degree of PR.
That LV preload may play an important role in determining exercise capacity is further supported by the observation that both RV and LV end-diastolic volumes - both indirect estimates of LV preload - showed positive correlation with Mets achieved and peak VO2, albeit not as strong a correlation as effective RVSVi. This appears to be counter-intuitive to the commonly held notion that increasingly dilated ventricular volumes equates with worsening exercise capacity. However, our results are by no means unique. There was a trend towards higher peak VO2 with increasing biventricular diastolic volumes in another study [20]. Similar to our findings, a recent study of 55 operated TOF patients found significant positive correlation between LVEDVi and peak work achieved [14]. They also found that exercise capacity between patients with and without RV dilation was similar. Supporting this notion, another study comparing treated pulmonary stenosis and TOF patients found similar exercise capacities and biventricular function in both groups, despite worse PR and larger RV volumes in TOF patients [22]. Our observations, as well as as those of other investigators, call into question the presumption that RV dilation necessarily means impaired exercise tolerance.
Questions have been raised regarding the use of RV size as a primary parameter in deciding the timing of PVR. Studies have found that exercise capacity and stroke volume are preserved late after tetrology repair, despite severe right ventricular dilation [14]. There are several postulations for this. Firstly, this may represent adaptive LV and RV remodeling in well-compensated TOF subjects. Of note, our TOF subjects had near-normal exercise capacities, indicating a good degree of compensation, despite their significant PR. Furthermore, our study has shown that exercise capacity correlates with stroke volumes, supporting the notion that proper adaptation to volume overload of RV could be helpful for preservation of functional capacity. This phenomenon is well recognized in left ventricular physiology among elite athletes [23, 24]. Secondly, PR results in a dilated but not hypertrophied RV. RV dilation exerts lesser impact on oxygen saturation and LV function, so long as RV stroke volume is maintained. Hence, exercise capacity is relatively well preserved [14]. This notion is supported by our observation that effective RV stroke volume is a significant determinant of exercise capacity. The potential of effective RVSVi as an indirect indicator of LV preload, and therefore cardiac output (in patients with largely normal LV function), raises the possibility of this parameter being used for decisions regarding the optimal timing of PVR.
In our study, we have shown that effective RVSVi significantly correlates with exercise capacity and maybe a potentially useful additional parameter in the decision for PVR. Nevertheless, the decision for timing of PVR is a complex one and should not be based primarily on a single parameter. Rather, each case should be considered individually: balancing the need to intervene before the point of irreversible remodeling against the likelihood for repeat procedures in future. The use of ventricular volumes alone may not take into account the degree of compensation of ventricular function. Many factors may also influence the poorer exercise performance in operated TOF patients: not only residual PR, but also residual right ventricular outflow tract obstruction, impaired biventricular function and presence of ventricular or atrial arrhythmias [25].
Abnormal cardiac function and and haemodynamic abnormalities secondary to residual PR and other residual defects may only appear after longer periods of follow-up. As time passes, PR leads eventually to worsening right ventricle enlargement and dysfunction, with consequent biventricular dyssynchrony, progression to heart failure, and poor performance at physical exercise. The challenge is to identify the optimal timing prior to irreversible decompensation for intervention. Presence of arryhythmia is another important consideration in the decision for PVR. Ventricular and atrial arrhythmias occur not uncommonly after correction of TOF and are one of the causes for sudden cardiac death in this group of patients [1, 26]. Both the ESC and CCS guidelines recommend PVR in the presence of sustained atrial or ventricular arrhythmias [10, 11]. In our cohort of repaired TOF patients, none had any malignant arrhythmias necessitating PVR.
The strength of our study lies in the unique set of data on exercise capacity in operated TOF patients with significant PR, which provides insights into the relationship between exercise capacity and CMR findings in this group of patients. The main limitations of our study is its retrospective and cross-sectional design, with lack of longitudinal CPET and CMR data. The longitudinal relationship between exercise capacity and biventricular volumes and function were not addressed and are best studied in a prospective cohort. The retrospective nature of our study also did not allow us to collect data on exercise habits and behavior which may be significant in this group of young patients and could better help explain our findings. Further, most subjects had near normal exercise capacity, which limits extrapolation of the findings to TOF cases with end-stage symptoms. Finally, our study had been performed in a single centre with relatively few patient number. This could have inadvertently introduced some bias and affect the generalizability of our results.
Conclusion
Although the majority of our TOF subjects had near normal exercise capacity; objective measures of Mets achieved, VO2max and peak oygen pulse were significant lower than controls. Increased PR fraction in TOF patients was associated with lower AT. Indexed effective RV stroke volume, which we postulate to be a measure of LV preload in the presence of significant PR, was positively associated with VO2max and Mets achieved. This may potentially be used as a predictor of exercise capacity in repaired TOF with significant PR.
Abbreviations
- TOF:
-
Tetralogy of Fallot
- RV:
-
Right ventricle
- CPET:
-
Cardiopulmonary exercise testing
- CMR:
-
Cardiac magnetic resonance imaging
- ACC?AHA:
-
American College of Cardiology/American Heart Asscoiation
- ESC:
-
European Society of Cardiology
- CCS:
-
Canadian Cardiovascular Society
- PVR:
-
Pulmonary valve replacement
- PR:
-
Pulmonary regurgitation
- EDV:
-
End diastolic volume
- ESV:
-
End systolic volume
- i:
-
indexed
- LV:
-
Left ventricle
- RVOT:
-
Right ventricular outflow tract
- EF:
-
Ejection fraction
- SV:
-
Stroke volume
- VO2:
-
Oxygen uptake
- VE:
-
Minute ventilation
- VCO2:
-
Carbon dioxide output
- AT:
-
Anaerobic threshold
- CI:
-
Confidence interval.
References
Murphy JG, Gersh BJ, Mair DD, Fuster V, McGoon MD, Ilstrup DM, McGoon DC, Kirklin JW, Danielson GK: Long-term outcome in patients undergoing surgical repair of tetralogy of fallot. N Engl J Med. 1993, 329: 593-599. 10.1056/NEJM199308263290901.
Zahka KG, Horneffer PJ, Rowe SA, Neill CA, Manolio TA, Kidd L, Gardner TJ: Long-term valvular function after total repair of tetralogy of Fallot: relation to ventricular arrhythmias. Circulation. 1988, 78 (suppl III): 14-19.
Redington AN, Oldershaw PJ, Shinebourne EA, Rigby ML: A new technique for the assessment of pulmonary regurgitation and its application to the assessment of right ventricular function before and after repair of tetralogy of Fallot. Br Heart J. 1988, 60: 57-65. 10.1136/hrt.60.1.57.
Gatzoulis MA, Till JA, Somerville J, Redington AN: Mechanoelectrical interaction in tetralogy of Fallot: QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995, 92: 231-237. 10.1161/01.CIR.92.2.231.
Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G: Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005, 95: 779-782. 10.1016/j.amjcard.2004.11.037.
Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJ: Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007, 116: 545-551. 10.1161/CIRCULATIONAHA.106.659664.
Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, Johansson B, Bouzas B, Mullen MJ, Poole-Wilson PA, Francis DP, Gatzoulis MA: Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005, 112: 828-835. 10.1161/CIRCULATIONAHA.104.529800.
Buys R, Van De Bruaene A, De Meester P, Budts W, Vanhees L: Predictors of mid-term event-free survival in adults with corrected tetralogy of Fallot. Acta Cardiol. 2012, 67: 415-421.
Giardini A, Specchia S, Tacy TA, Coutsoumbas G, Gargiulo G, Donti A, Formigari R, Bonvicini M, Picchio FM: Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol. 2007, 99: 1462-1467. 10.1016/j.amjcard.2006.12.076.
Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG): ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010, 31: 2915-2957.
Silversides CK, Kiess M, Beauchesne L, Bradley T, Connelly M, Niwa K, Mulder B, Webb G, Colman J, Therrien J: Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan's syndrome. Can J Cardiol. 2010, 26: e80-e97. 10.1016/S0828-282X(10)70355-X.
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, et al: ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thora/cic Surgeons. J Am Coll Cardiol. 2008, 52: e143-e263. 10.1016/j.jacc.2008.10.001.
Dave HH, Buechel ER, Dodge-Khatami A, Kadner A, Rousson V, Bauersfeld U, Prêtre R: Early insertion of a pulmonary valve for chronic regurgitation helps restoration of ventricular dimensions. Ann Thorac Surg. 2005, 80: 1615-1620. 10.1016/j.athoracsur.2005.04.058. discussion 1620–1
O'Meagher S, Munoz PA, Alison JA, Young IH, Tanous DJ, Celermajer DS, Puranik R: Exercise capacity and stroke volume are preserved late after tetralogy repair, despite severe right ventricular dilatation. Heart. 2012, 98: 1595-1599. 10.1136/heartjnl-2012-302147.
Ghez O, Tsang VT, Frigiola A, Coats L, Taylor A, Van Doorn C, Bonhoeffer P, De Leval M: Right ventricular outflow tract reconstruction for pulmonary regurgitation after repair of tetralogy of Fallot. Preliminary results. Eur J Cardiothorac Surg. 2007, 31: 654-658. 10.1016/j.ejcts.2006.12.031.
Coats L, Khambadkone S, Derrick G, Hughes M, Jones R, Mist B, Pellerin D, Marek J, Deanfield JE, Bonhoeffer P, Taylor AM: Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. Eur Heart J. 2007, 28: 1886-1893. 10.1093/eurheartj/ehm181.
Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP: Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life–single centre experience and review of published data. Eur Heart J. 2012, 33: 1386-1396. 10.1093/eurheartj/ehr461.
Wasserman K: The anaerobic threshold: definition, physiological significance and identification. Adv Cardiol. 1986, 35: 1-23.
Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, Schneider S, Schwarz A, Senges J: Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002, 106: 3079-3084. 10.1161/01.CIR.0000041428.99427.06.
Kempny A, Diller GP, Orwat S, Kaleschke G, Kerckhoff G, Bunck AC, Maintz D, Baumgartner H: Right ventricular-left ventricular interaction in adults with Tetralogy of Fallot: a combined cardiac magnetic resonance and echocardiographic speckle tracking study. Int J Cardiol. 2012, 154: 259-264. 10.1016/j.ijcard.2010.09.031.
Spiewak M, Małek LA, Petryka J, Mazurkiewicz L, Marczak M, Biernacka EK, Kowalski M, Hoffman P, Demkow M, Miśko J, Rużyłło W: Determinants of left- and right ventricular ejection fractions in patients with repaired tetralogy of Fallot: a cardiac magnetic resonance imaging study. Pol Arch Med Wewn. 2013, 123: 539-546.
Luijnenburg SE, de Koning WB, Romeih S, van den Berg J, Vliegen HW, Mulder BJ, Helbing WA: Exercise capacity and ventricular function in patients treated for isolated pulmonary valve stenosis or tetralogy of Fallot. Int J Cardiol. 2012, 158: 359-363. 10.1016/j.ijcard.2011.01.038.
Pelliccia A, Culasso F, Di Paolo FM, Maron BJ: Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999, 130: 23-31. 10.7326/0003-4819-130-1-199901050-00005.
Barbier J, Ville N, Kervio G, Walther G, Carré F: Sports-specific features of athlete's heart and their relation to echocardiographic parameters. Herz. 2006, 31: 531-543. 10.1007/s00059-006-2862-2.
Bassareo PP, Saba L, Solla P, Barbanti C, Marras AR, Mercuro G: Factors influencing adaptation and performance at physical exercise in complex congenital heart diseases after surgical repair. Biomed Res Int. 2014, 2014: 862372-
Harrison DA, Harris L, Siu SC, MacLoghlin CJ, Connelly MS, Webb GD, Downar E, McLaughlin PR, Williams WG: Sustained ventricular tachycardia in adult patients late after repair of tetralogy of Fallot. J Am Coll Cardiol. 1997, 30: 1368-1373. 10.1016/S0735-1097(97)00316-1.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2261/14/122/prepub
Acknowledgements
The authors would like to acknowledge the efforts of the nurses of the cardiopulmonary exercise testing unit for helping to collate the data of the cardiopulmonary exercise testing. The authors have no sources of funding to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JY was involved in the design of the project, acquisition of data, analysis of the results and drafting of the manuscript. JLT, SYT and RST were involved in the design of the project, analysis of the results and drafting of the manuscript. TTL, FG, LZ and RL were involved in the acquisition of data, analysis of results and revision of the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yap, J., Le Tan, J., Le, T.T. et al. Assessment of left ventricular preload by cardiac magnetic resonance imaging predicts exercise capacity in adult operated tetralogy of Fallot: a retrospective study. BMC Cardiovasc Disord 14, 122 (2014). https://doi.org/10.1186/1471-2261-14-122
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2261-14-122