Abstract
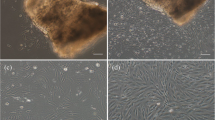
A cell culture of rare and threatened species of Sakhalin sturgeon Acipenser mikadoi was established from a fragment of the pectoral fin and neighboring tissues. Initially, the culture consisted of different cell types including typical fibroblasts as well as cells of epithelial origin, myofibroblasts, etc. After approximately five passages, the culture largely consisted of cells with fibroblast morphology. Under normal culture conditions, these cells grew for more than one year at a constant rate and passed about 80 population doublings. In the absence of serum, cells entered the state of proliferative quiescence (G0-state). When cultured without medium replacement for a long period of time, cells fused to form myofibers of about 1 cm in length. These myofibers could branch and acquired cross striation with time. About forty days after myofibers emerged, they degenerated, lost their shape, detached from the substrate, and finally died. The induction of adipogenic differentiation arrested cell proliferation and introduced lipophilic inclusions formed in a minor fraction of cells. The number of these inclusions was low, and cells with inclusions demonstrated various morphology distinct from typical adipocytes. The induction of osteogenic differentiation gave rise to cells that produce mineralized extracellular matrix and bone nodules. Chromosome analysis revealed a set of chromosomes typical for “high chromosome” sturgeon species. The variation in the chromosome number was very high (mean, 247 ± 33; modal value, 248). The analysis involving AT- and GC-specific fluorochromes has demonstrated that the telomeric and centromeric regions of all chromosomes are enriched in GC content. The distribution of AT- and GC-rich sequences along the chromosomes was heterogeneous. Long chromosomes were preferentially stained by the AT-specific dye, whereas small chromosomes demonstrated brighter fluorescence after 7-amino-actinomycin D staining; in particular, several small chromosomes fluoresced extremely brightly. This work is the first report of cell culture and karyotype analysis of Sakhalin sturgeon.
Similar content being viewed by others
References
Omoto, N., Maebayashi, M., Hara, A., Adashi, S., and Yamauchi, K., Gonadal Maturity in Wild Sturgeons, Huso dauricus, Acipenser mikadoi and A. schrenckii Caught Near Hokkaido, Japan. Environmental Biology of Fishes, 2004, vol. 70, pp. 381–391.
Lyubaev, V.Ya., Brood Stock of Sakhalin (Green) Sturgeon As a Basic Gene Pool for Species Conservation, International Conference “Conservation of Genetic Resources,” St. Petersburg, October 19–22, 2004, pp. 812–813.
Drokin, S.I. and Kopeika, E.F., Motility of and Phospholipid Content in Cryopreserved Spermatozoa of Three Sturgeon Species, Sturg. Quart., 1996, vol. 4, pp. 8–10.
Tsvetkova, L.I., Dokina, O.B., Pronina, N.D., and Milenko, V.A., Cryopreservation of Fish Sperm: Current State, Development, and Outlooks in Izbrannye trudy VNIIPRKh (v 4 tomakh), (Selected Works of VNIIPRKh (in 4 vols)), Book 1, vol. I–II, Dmitrov, Sever Podmoskov’ya, 2002, pp. 358–365.
Birstein, V.J., Poletaev, A.I., and Goncharov, B.F., DNA Content in Eurasian Sturgeon Species Determined by Flow Cytometry, Cytometry, 1993, vol. 14, pp. 377–383.
Birstein, V.J., Hanner, R., and Desalle, R., Phylogeny of the Acipenseriformes: Cytogenetic and Molecular Approaches, Environmental Biology of Fishes, 1997, vol. 48, pp. 127–155.
Wang, G., Lapatra, S., Zeng, L., Zhao, Z., and Lu, Y., Establishment, Growth, Cryopreservation and Species of Origin Identification of Three Cell Lines from White Sturgeon, Acipenser transmontanus, Methods Cell Sci., 2003, vol. 25, pp. 211–220.
Li, M.F., Marrayatt, V., Annand, C., and Odense, P., Fish Cell Cultures: Two Newly Developed Cell Lines from Atlantic Sturgeon (Acipenser oxyrhynchus) and Guppy (Poecilia reticulate), Can. J. Zool., 1985, vol. 63, pp. 2867–2874.
Watson, L.R., Groff, J.M., and Hedrick, R.P., Replication and Pathogenesis of White Sturgeon Iridovirus (WSIV) in Experimentally Infected White Sturgeon Acipenser transmontanus Juveniles and Sturgeon Cell Lines, Dis. Aquat. Org., 1998, vol. 32, pp. 173–184.
Artyukhin, E.N. and Andronov, A.E., Some Features of the Sturgeon in Tumnin River, Osetrovoe Khozyaistvo Vodoemov SSSR (Sturgeon Industry in the USSR Water Bodies), Tez. Dokl., part 1, Astrakhan’, 1989, pp. 9–10.
Artyukhin, E.N. and Andronov, A.E., Morphobiological Description of Green Sturgeon Acipenser medirostris (Chondrostei, Acipenseridae) from Tumnin (Datta) River and Some Aspects of Ecology and Zoogeography of Sturgeons. Zoologicheskii Zhurnal (Rus.), 1990, vol. 69, pp. 81–91.
Mikodina, E.V., Khrisanfov, V.E., Lyubaev, V.Ya., and Presnyakov, A.V., Artificial Spawning of Sakhalin Sturgeon in Russia, International Conference “Aqua-2006,” Florence, Italy, May 9–13, 2006, p. 610.
Aljanabi, S.M. and Martinez, I., Universal and Rapid Salt-Extraction of High Quality Genomic DNA for PCR-Based Techniques, Nucleic Acids Research, 1997, vol. 25, pp. 4692–4693.
Mugue, N.S., Barmintseva, A.E., Rastorguev, S.M., Mugue, V.N., and Barmintsev, V.A., Polymorphism of the Mitochondrial DNA Control Region in Eight Sturgeon Species and Development of a System for DNA-Based Species Identification, Genetika, 2008, vol. 44, pp. 913–919.
Ayres, W.O., Descriptions of Three New Species of Sturgeon from San Francisco, Proc. California Acad. Natural Sci., 1854, vol. 1, pp. 14–15 (1854–1857).
Hilgendorf, F., Uber Eine Neue Stor-Art Aus Nord-Japan (Acipenser mikadoi), Sitzungsber. Ges. Naturf. Freunde (Berlin), 1892, pp. 142–144.
Elkins, C., Miller, J., and Kaplan, J., Petition to List the North American Green Sturgeon (Acipenser medirostris) As an Endangered or Threatened Species under the Endangered Species Act, Environmental Protection Information Center, Center for Biological Diversity Waterkeepers Northern California Petitioners, June 2001 ( http://www.biologicaldiversity.org/species/fish/North_American_green_sturgeon/pdfs/PETITION.PDF ).
Birstein, V.J. and Bemis, W.E., How Many Species Are There within the Genus Acipenser? Environmental Biology of Fishes, 1997, vol. 48, pp. 157–163.
Birstein, V.J. and Desalle, R., Molecular Phylogeny of Acipenserinae, Molecular Phylogenetics and Evolution, 1998, vol. 9, pp. 141–155.
Prasanna, I., Lakra, W.S., Ogale, S.N., and Bhonde, R.R., Cell Culture from Fin Explants of Endangered Golden Mahseer, Tor putitora (Hamilton), Current Science, 2000, vol. 79, pp. 93–95.
Alvarez, M.C., Bejar, J., Chen, S., and Hong, Y., Fish ES Cells and Application to Biotechnology, Marine Biotechnology, 2006, vol. 9, pp. 117–127.
Fantana, F., Lanfredi, M., Rossi, R., Bronzi, P., and Arlati, G., Established Cell Lines from Three Sturgeon Species, Sturg. Quart., 1995, vol. 3, pp. 6–7.
Hedrick, R.P., McDowell, T.S., Rosenmark, R., Aronstein, D., and Lannan, C.N., Two Cell Lines from White Sturgeon, Trans. Amer. Fish. Soc., 1991, vol. 120, pp. 528–534.
Li, M.F., Marrayatt, V., Annand, C., and Odense, P., Fish Cell Culture: Two Newly Developed Cell Lines from Atlantic Sturgeon (Acipenser oxyrhynchus) and Guppy (Poecilia reticulate). Canad. J. Zool., 1985, vol. 63, pp. 2867–2874.
Ramirez, R.D., Morales, C.P., Herbert, B.-S., Rohde, J.M., Passons, C., Shay, J.W., and Wright, W.E., Putative Telomere-Independent Mechanisms of Replicative Aging Reflect Inadequate Growth Conditions, Genes Dev., 2001, vol. 15, pp. 398–403.
Dashinimaev, E.B., Vishnyakova, K.S., Popov, K.V., and Yegorov, Y.E., Stable Culture of hTERT-Transduced Human Embryonic Neural Stem Cells Holds All the Features of Primary Culture, Electronic Journal of Biology, 2008, vol. 4, pp. 93–97.
Shima, F., Nikaido, O., Shinohara, S., and Egami, N., Continued in Vitro Growth of Fibroblast-Like Cells (RBCF-1) Derived from the Caudal Fin of the Fish, Carassius auratus. Exp. Gerontol., 1980, vol. 15, pp. 305–314.
Panyukhin, N.V., Vishnyakova, K.S., and Yegorov, Y.E., Effect of Partial Oxygen Pressure on Survival, Proliferation and Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells, Biologicheskie Membrany, 2008, vol. 25, pp. 260–267 [Biochemistry (Moscow) Suppl. Ser. A: Membrane and Cell Biol. (Engl. Transl.), 2008, vol. 2, pp. 326–332].
Nowruzfashkhami, M.R., Safaiian, S., Bahmani, M., and Chubian, F., Karyotype Analysis in Ship Sturgeon Acipenser nudiventris in the South Caspian Sea Using Leukocyte Culture, J. Appl. Ichthyol., 2006, vol. 22, pp. 97–98.
Fontana, F., Congiu, L., Mudrak, V.A., Quattro, J.M., Smith, T.I.J., Ware, K., and Doroshov, S.I., Evidence of Hexaploid Karyotype in Shortnose Sturgeon, Genome, 2008, vol. 51, pp. 113–119.
Fontana, F., Tagliavini, J., Congiu, L., Lanfredi, M., Chicca, M., Laurenti, C., and Rossi, R., Karyotypic Characterization of the Great Sturgeon, Huso huso, by Multiple Staining Techniques and Fluorescent in Situ Hybridization, Mar. Biol., 1998, vol. 132, pp. 495–501.
Tagliavini, J., Williot, P., Congiu, L., Chicca, M., Lanfredi, M., Rossi, R., and Fontana, F., Molecular Cytogenetic Analysis of the Karyotype of the European Atlantic Sturgeon, Acipenser sturio, Heredity, 1999, vol. 83, pp. 520–525.
Chicca, M., Suciu, R., Ene, C., Lanfredi, M., Congiu, L., Leis, M., Tagliavini, J., Rossi, R., and Fontana, F., Karyotype Characterization of the Stellate Sturgeon, Acipenser stellatus, by Chromosome Banding and Fluorescent in Situ Hybridization, J. Appl. Ichthyol., 2002, vol. 18, pp. 298–300.
Fontana, F., Lanfredi, M., Chicca, M., Congiu, L., Tagliavini, J., and Rossi, R., Fluorescent in Situ Hybridization with rDNA Probes on Chromosomes of Acipenser ruthenus and Acipenser naccarii (Osteichthyes Acipenseriformes), Genome, 1999, vol. 42, pp. 1008–1012.
Fontana, F., Lanfredi, M., Kirschbaum, F., Garrido-Ramos, M.A., Robles, F., Forlani, A., and Congiu, L., Comparison of Karyotypes of Acipenser oxyrinchus and A. sturio by Chromosome Banding and Fluorescent in Situ Hybridisation, Genetica, 2008, vol. 132, pp. 281–286.
Fontana, F., Chromosomal Nucleolar Organizer Regions in Four Sturgeon Species As Markers of Karyotype Evolution in Acipenseriformes (Pisces), Genome, 1994, vol. 37, pp. 888–892.
Van Eenennaam, A.L., Murray, J.D., and Medrano, J.F., Karyotype of the American Green Sturgeon, Transactions of the American Fisheries Society, 1999, vol. 128, pp. 175–177.
Nowruzfashkhami, M.R., Pourkazemi, M., and Baradarannoveiri, S., Chromosome Study of Persian Sturgeon Acipenser persicus B., Cytologia, 2000, vol. 65, pp. 197–202.
Fontana, F., Lanfredi, M., Rossi, R., Bronzi, P., and Arlati, G., Karyotypic Characterization of Acipenser gueldenstaedti with C-, AgNO3 and Fluorescence Banding Techniques, Ital. J. Zool., 1996, vol. 63, pp. 113–118.
Fontana, F., Bruch, R.M., Binkowski, F.P., Lanfredi, M., Chicca, M., Beltrami, N., and Congiu, L., Karyotype Characterization of the Lake Sturgeon, Acipenser fulvescens (Rafinesque, 1817) by Chromosome Banding and Fluorescent in situ Hybridization, Genome, 2004, vol. 47, pp. 742–746.
Yu X., Zhou T., Li K., Li Y., Zhou M. On the Karyosystematics of Cyprinid Fishes and a Summary of Fish Chromosome Studies in China, Genetica, 1987, vol. 72, pp. 225–236.
Van Eenennaam, A.L., Murray, J.D., and Medrano, J.F., Mitotic Analysis of the North American White Sturgeon, Acipenser transmontanus Richardson (Pisces, Acipenseridae), a Fish with a Very High Chromosome Number, Genome, 1998, vol. 41, pp. 266–271.
Kim, D.S., Nam, Y.K., Noh, J.K., Park, C.H., and Chapman, F.A., Kariotype of North American Shortnose Sturgeon Acipenser brevirostrum with the Highest Chromosome Number in the Acipenseriformes, Ichthyological Res., 2005, vol. 52, pp. 94–97.
Kim, E.S., Punina, E.O., and Rodionov, A.V., Chromosome CPD(PI/DAPI)- and CMA/DAPI-banding Patterns in Allium cepa L., Genetika (Rus.), 2002, vol. 38, pp. 489–496.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.S. Vishnyakova, N.S. Mugue, D.A. Zelenina, E.V. Mikodina, O.A. Kovaleva, G.V. Madan, Y.E. Yegorov, 2008, published in Biologicheskie Membrany, 2008, Vol. 25, No. 6, pp. 420–433.
Rights and permissions
About this article
Cite this article
Vishnyakova, K.S., Mugue, N.S., Zelenina, D.A. et al. Cell culture and karyotype of Sakhalin sturgeon Acipenser mikadoi . Biochem. Moscow Suppl. Ser. A 3, 42–54 (2009). https://doi.org/10.1134/S1990747809010061
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747809010061