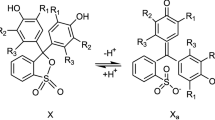
Abstract
Acid-base properties of 4-hydroxystyryl dyes in aqueous solution have been studied by means of chemical tristimulus colorimetry and spectrophotometry. Ionization and hydroxylation constants of the dyes have been determined using chromaticity functions of specific and CIE color difference. It has been shown that aggregation processes in the solution of 4-hydroxystyryl dyes can be observed by means of tristimulus colorimetry. A scheme of acid-base transformations of the studied dyes in the solution has been suggested, and selected spectrophotometric parameters of the equilibrium ionic and molecular forms have been determined.
Similar content being viewed by others
References
Kulinich, A.V. and Ishchenko, A.A., Russ. Chem. Rev., 2009, vol. 78, no. 2, p. 141. doi 10.1070/RC2009v078n02ABEH003900
Merocyanines: Synthesis and Application. Heterocyclic Polymethine Dyes, Strekowsky, L., Ed., Berlin; Heidelberg: Springer-Verlag, 2008, p. 75. doi 10.1007/7081_2007_110.
Brooker, L.G.S., Keyes, G.H., and Heseltine, D.W., J. Am. Chem. Soc., 1951, vol. 73, no. 11, p. 5350. doi 10.1021/ja01155a096
Spiropyran Leuco Dyes, Muthyala, R., ed., New York; Boston; Dordrecht; London; Moscow: Kluwer Academic Publishers, 2002, p. 1. doi 10.1007/0-306- 46906-5_1
Jeong, J.H., Kang, B.J., Kim, J.S., Jazbinsek, M., Lee, S.H., Lee, S.C., Baek, I.H., Yun, H., Kim, J., Lee, Y.S., Lee, J.H., Kim, J.H., Rotermund, F., and Kwon, O.P., Sci. Rep., 2013, no. 3, p. 3200. doi 10.1038/srep03200
Van Bezouw, S., Campo, J., Lee, S.H., Kwon, O.P., Wenseleers, W., J. Phys. Chem. (C), 2015, vol. 119, no. 37, p. 21658. doi 10.1021/acs.jpcc.5b06968
Gasiorowska, M., Typek, J., Soroka, J.A., Sawicka, M.J., Wróblewska, E.K., Guskos, N., and Zolnierkiewicz, G., Spectrochim. Acta Mol. Biomol. Spectrosc., 2014, vol. 124, p. 300. doi 10.1016/j.saa.2014.01.007
Zheng, X., Zhu, W., Liu, D., Ai, H., Huang, Y., and Lu, Z., ACS Appl. Mater. Interfaces., 2014, vol. 6, no. 11, p. 7996. doi 10.1021/am501546h
Ren, W.X., Han, J., Pradhan, T., Lim, J.Y., Lee, J.H., Lee, J., Kim, J.H., and Kim, J.S., Biomaterials, 2014, vol. 35, no. 13, p. 4157. doi 10.1016/j.biomaterials. 2014.01.055
Kovtun, Y.P., Prostota, Y.O., Shandura, M.P., Poronik, Y.M., and Tolmachev, A.I., Dyes and Pigments, 2004, vol. 60, p. 215. doi 10.1016/S0143-7208(03)00152-9
Linn, M.M., Poncio, D.C., and Machado, V.G., Tetrahedron Lett., 2007, vol. 48, no. 26, p. 4547. doi 10.1016/j.tetlet.2007.04.141
Reichardt, C., Pure Appl. Chem., 2008, vol. 80, no. 7, p. 1415. doi 10.1351/pac200880071415
Fidale, L.C., Heinze, T., and El Seoud, O.A., Carbohyd. Polym., 2013, vol. 93, no. 1, p. 129. doi 10.1016/j.carbpol.2012.06.061
Nandi, L.G., Nicoleti, C.R., Bellettini, I.C., and Machado, V.G., Anal. Chem., 2014, vol. 86, no. 10, p. 4653. doi 10.1021/ac501233x
Krieg, R., Eitner, A., Günther, W., and Halbhuber, K.J., Biotech. Histochem., 2007, vol. 82, nos. 4–5, p. 235. doi 10.1080/10520290701714013
Prukala, D., Prukala, W., Gierszewski, M., Karolczak, J., Khmelinskii, I., Sikorski, M., Dyes and Pigments., 2014, vol. 108, p. 126. doi 10.1016/j.dyepig. 2014.04.013
Prukala, D., Prukala, W., Gierszewski, M., Karolczak, J., Khmelinskii, I., and Sikorski, M., Photochem. Photobiol. Sci., 2012, vol. 11, no. 9, p. 1454. doi 10.1039/C2PP25063B
Ivanov, V.M. and Kuznetsova, O.V., Russ. Chem. Rev., 2001, vol. 70, no. 5, p. 357. doi 10.1070/RC2001v070n05ABEH000636
Chebotarev, A.N., Snigur, D.V., Bevziuk, K.V., and Efimova, I.S., Metodyi Ob”ekty Khim. Analiza, 2014, vol. 9, no. 1, p. 4.
Chebotarev, A.N. and Snigur, D.V., J. Analyt. Chem., 2015, vol. 70, no. 1, p. 55. doi 10.1134/S1061934815010062
Ivanov, V.M., Monogarova, O.V., and Oskolok, K.V., J. Analyt. Chem., 2015, vol. 70, no. 10, p. 1165. doi 10.1134/S1061934815100111
Chebotarev, A.N. and Snigur, D.V., Russ. J. Gen. Chem., 2016, vol. 86, no. 4, p. 815. doi 10.1134/S1070363216040095
Sayama, K., Tsukagoshi, S., Hara, K., Ohga, Y., Shinpou, A., Abe, Y., and Arakawa, H., J. Phys. Chem. (B), 2002, vol. 106, no. 6, p. 1363. doi 10.1021/jp0129380
Egorov, V.V. and Alfimov, M.V., Phys. Usp., 2007, vol. 50, no. 10, p. 985. doi 10.3367/UFNr.0177.200710a.1033
Zhukova, Yu.P. and Studenyak, Ya.A., Nauk. Vsn. Uzhgorod. Univ., Ser. Kh?m., 2014, no. 2(32), p. 38.
Zhukova, Yu.P. and Studenyak, Ya.A., Nauk. Vsn. Uzhgorod. Univ., Ser. Kh?m., 2015, no. 2(34), p. 40.
Wang, M., Gao, M., Miller, K.D., Sledge, G.W., Hutchins, G.D., and Zheng, Q.H., Eur. J. Med. Chem., 2009, vol. 44, no. 5, p. 2300. doi 10.1016/j.ejmech.2008.02.033
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Chebotarev, D.V. Snigur, Yu.P. Zhukova, K.V. Bevziuk, Ya.I. Studenyak, Ya.R. Bazel, 2017, published in Zhurnal Obshchei Khimii, 2017, Vol. 87, No. 2, pp. 216–224.
Rights and permissions
About this article
Cite this article
Chebotarev, A.N., Snigur, D.V., Zhukova, Y.P. et al. Tristimulus colorimetric and spectrophotometric study of the state of 4-hydroxystyryl dyes in aqueous solutions. Russ J Gen Chem 87, 196–203 (2017). https://doi.org/10.1134/S1070363217020074
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363217020074