Abstract
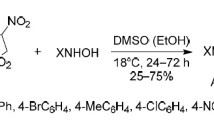
3-Aryl-6а-methyl-6-nitro-1-carbamoylhexahydrothieno[2,3-d]pyrazole-4,4-dioxides, novel original bicyclic species consisting of fused pyrazolidine and sulfolane rings, and 1,4-adducts were obtained by reacting 2-benzylidene-3-methyl-4-nitro-3-thiolene-1,1-dioxide and its derivatives with semicarbazide.
Similar content being viewed by others
References
Brant, M.G. and Wulff, J.E., Org. Lett., 2012, vol. 14, p. 5876. DOI: 10.1021/ol3027939.
Eid, A.A., Koubeissi, A., Bou-Mjahed, R., Al Khalil, N., Farah, M., Maalouf, R., Nasser, N., and Bouhadir, K.H., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 174. DOI: 10.1016/j.bmcl.2012.10.122.
Kiriazis, A., af Gennäs, G.B., Talman, V., Ekokoski, E., Ruotsalainen, T., Kylänlahti, I., Ruffer, T., Wissel, G., Xhaard, H., Lang, H., Tuominen, R.K., and Yli-Kauhaluoma, J., Tetrahedron, 2011, vol. 67, p. 8665. DOI: 10.1016/j.tet.2011.09.044.
Li, B., Buzon, R.A., and Hritzko, B., Synlett, 2012, vol. 23, p. 131. DOI: 10.1055/s-0031-1290089.
Oh, K., Org. Lett., 2007, vol. 9, p. 2973. DOI: 10.1021/ol0710663.
Tolstikov, G.A., Shul’ts, E.E., Vafina, G.F., Spirikhin, L.V., Zh. Org. Khim., 1992, vol. 28, no. 2, p.190.
Waser, M., Moher, E.D., Borders, S.S.K., Hansen, M.M., Hoard, D.W., Laurila, M.E., LeTourneau, M.E., Miller, R.D., Phillips, M.L., Sullivan, K.A., Ward, J.A., Xie, C., Bye, C.A., Leitner, T., Herzog-Krimbacher, B., Kordian, M., and Mullner, M., Org. Proc. Res. Dev., 2011, vol. 15, p. 1266. DOI: 10.1021/op100325h.
Zarovnaya, I.S., Zlenko, H.T., and Palchikov, V.A., Eur. Chem. Bull., 2014, vol. 3, p.543.
Wong, S.S.Y., Brant, M.G., Barr, C., Oliver, A.G., and Wulff, J.E., Beilstein J. Org. Chem., 2013, vol. 9, p. 1419. DOI: 10.3762/bjoc.9.159.
Gore, P.M., Hancock, A.P., Hodgson, S.T., Procopiou, P.A., and Vile, S., WO Patent 2009021965 A3, 2009.
Berestovitskaya, V.M., Zh. Obshch. Khim., 2000, vol. 70, no. 9, p. 1512.
Berestovitskaya, V.M., Selivanova, M.V., Vakulenko, M.I., Efremova, I.E., and Berkova, G.A., Russ. J. Org. Chem., 2009, vol. 45, no. 12, p. 1814. DOI: 10.1134/S1070428009120100.
Berestovitskaya, V.M., Efremova, I.E., Lapshina, L.V., Serebryannikova, A.V., Gurzhiy, V.V., and Abzianidze, V.V., Mendeleev Commun., 2015, vol. 25, p. 191. DOI: 10.1016/j.mencom.2015.05.010.
Ziyaei-Halimehjani, A., Namboothiri, I.N.N., and Hooshmand, S.E., RSC Adv., 2014, vol. 4, p. 48022. DOI: 10.1039/C4RA08828J.
Ziyaei-Halimehjani, A., Namboothiri, I.N.N., and Hooshmand, S.E., RSC Adv., 2014, vol. 4, p. 31261. DOI: 10.1039/C4RA04069D.
Lapshina, L.V., Serebryannikova, A.V., Efremova, I.E., Perkhunova, A.D., Bortnikov, S.V., and Berestovitskaya, V.M., Russ. J. Gen. Chem., 2014, vol. 84, no. 8, p. 1519. DOI: 10.1134/S10703632140801.
Berestovitskaya, V.M., Efremova, I.E., Trukhin, E.V., and Berkova, G.A., Zh. Org. Khim., 1993, vol. 29, no. 2, p.368.
Baldwin, J.E., J. Chem. Soc. Chem. Commun., 1976, p.734.
Argyle, C.S., Goadby, S.C., Mason, K.G., Reed, R.A., Smith, M.A., and Stern, E.S., J. Chem. Soc. (C), 1967, vol. 21, p. 2156.
Pretch, E., Bühlmann, P., and Affolter, C., Structure Determination of Organic Compounds: Tables of Spectral Data, Berlin: Springer, 2000.
Spek, A.L., PLATON, A Multipurpose Crystallographic Tool. Utrecht University, Utrecht, The Netherlands, 2005.
Sheldrick, G.M., SADABS, University of Göttingen, Göttingen, Germany, 2004.
Sheldrick, G.M., Acta Crystallogr. (A), 2008, vol. 64, p. 112. DOI: 10.1107/S0108767307043930.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H., J. Appl. Crystallogr., 2009, vol. 42, p. 339. DOI: 10.1107/S0021889808042726.
Vasil’eva, M.V., Berestovitskaya, V.M., Berkova, G.A., and Pozdnyakov, V.P., Zh. Org. Khim., 1986, vol. 22, p. 428.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.E. Efremova, A.V. Serebryannikova, L.V. Lapshina, V.V. Gurzhiy, V.M. Berestovitskaya, 2016, published in Zhurnal Obshchei Khimii, 2016, Vol. 86, No. 3, pp. 481–488.
To the 100th Anniversary of A.N. Pudovik
Rights and permissions
About this article
Cite this article
Efremova, I.E., Serebryannikova, A.V., Lapshina, L.V. et al. Synthesis of new bicyclic compounds containing fused sulfolane and pyrazolidine rings. Russ J Gen Chem 86, 622–628 (2016). https://doi.org/10.1134/S1070363216030191
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363216030191