Abstract
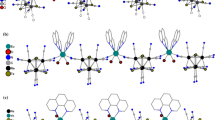
Two new porous coordination polymers based on cluster anions [Re4Te4(CN)12]4– and cationic Ln3+ (Ln = La, Gd) complexes with 1,10-phenanthroline (Рhen) are synthesized under hydrothermal conditions. The structures of the compounds are determined by X-ray diffraction analysis (CIF files CCDC 1437445 (I) and 1437446 (II)). Compound (РhenH)[{La(H2O)3(Рhen)2}{Re4Te4(CN)12}] · 1.5Рhen · 6H2O (I) crystallizes in the space group \(P\bar 1\) (triclinic system): a = 13.322(3), b = 15.977(3), c = 18.576(4) Å, α = 71.34(3)°, β = 85.56(3)°, γ = 88.27(3)°, V = 3734.8(13) Å3. Compound (PhenH)[{Gd(H2O)2(Phen)2}{Re4Te4(CN)12}] · 2Phen · 0.5H2O (II) crystallizes in the space group C2/c (monoclinic crystal system): a = 18.146(1), b = 30.245(2), c = 13.455(2) Å, β = 97.858(2)°, V = 7315.4(1) Å3. Structures I and II are based on polymer chains consisting of alternating fragments [Re4Te4(CN)12]4– and {Ln(H2O) n (Phen)2}3+ (Ln = La, n = 3; Ln = Gd, n = 2) linked by the bridging CN ligands. The packings of the polymers contain extended channels due to the developed network of noncovalent interactions. The walls of the channels are formed by both hydrophilic (CN–) and hydrophobic (Рhen) groups. The channels, whose volume is 25 and 15% for compounds I and II, respectively, are filled by disordered Phen molecules and PhenH+ cations, as well as by H2O molecules.
Similar content being viewed by others
References
Batten, S.R., Newille, S.M., and Turner, D.R., Coordination Polymers: Design, Analysis and Application, Cambridge: (UK): Royal Society of Chemistry, 2009.
Janiak, C. and Vieth, J.K., New J. Chem., 2010, vol. 34, no. 11, p. 2366.
Phan, A., Doonan, C.J., Uribe-romo, F.J., et al., Acc. Chem. Res., 2010, vol. 43, no. 1, p. 58.
Adatoz, E., Avci, A.K., and Keskin, S., Sep. Purif. Technol., 2015, vol. 152, p. 207.
Yaghi, O.M., O’Keeffe, M., Ockwig, N.W., et al., Nature, 2003, vol. 423, no. 6941, p. 705.
Eddaoudi, M., Kim, J., Rosi, N., et al., Science, 2002, vol. 295, no. 5554, p. 469.
Tranchemontagne, D.J., Mendoza-Cortez, J.L., O’Keeffe, M., and Yaghi, O.M., Chem. Soc. Rev., 2009, vol. 38, no. 5, p. 1257.
Kim, Y., Fedorov, V.E., and Kim, S.-J., J. Mater. Chem., 2009, vol. 19, no. 39, p. 7178.
Fedorov, V.E., Naumov, N.G., Mironov, Y.V., et al., J. Struct. Chem., 2002, vol. 43, no. 4, p. 721.
Naumov, N.G., Virovets, A.V., and Fedorov, V.E., J. Struct. Chem., 2000, vol. 41, no. 3, p. 609.
Laing, M., Kiernan, P.M., and Griffith, W.P., J. Chem. Soc., Chem. Commun., 1977, vol. 7, p. 221.
Mironov, Y.V., Virovets, A.V., Artemkina, S.B., and Fedorov, V.E., J. Struct. Chem., 1999, vol. 40, no. 2, p. 375.
Efremova, O.A., Mironov, Y.V., and Fedorov, V.E., Eur. J. Inorg. Chem., 2006, vol. 2006, no. 13, p. 2533.
Li, B., Wen, H.-M., Cui, Y., et al., Prog. Polym. Sci., 2015, vol. 48, p. 40.
Efremova, O.A., Mironov, Y.V., Kuratieva, N.V., and Fedorov, V.E., Polyhedron, 2011, vol. 30, no. 8, p. 1404.
Efremova, O.A., Golenkov, E.O., Mironov, Y.V., et al., J. Phys. Chem. C, 2007, vol. 111, no. 29, p. 11008.
Zhang, T. and Lin, W., Chem. Soc. Rev., 2014, vol. 43, no. 16, p. 5982.
Efremova, O.A., Gayfulin, Y.M., Mironov, Y.V., et al., Polyhedron, 2012, vol. 31, no. 1, p. 515.
APEX2 (version 1.08), SAINT (version 7.03), SADABS (version 2.11). Bruker Advanced X-ray Solutions, Madison: Bruker AXS Inc., 2004.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Original Russian Text © Ya.M. Gayfulin, N.V. Kuratieva, Yu.M. Litvinova, Yu.V. Mironov, 2016, published in Koordinatsionnaya Khimiya, 2016, Vol. 42, No. 7, pp. 387–392.
Rights and permissions
About this article
Cite this article
Gayfulin, Y.M., Kuratieva, N.V., Litvinova, Y.M. et al. Syntheses and structures of 1d coordination polymers based on cluster anions [Re4Te4(CN)12]4– and cationic Ln3+ (Ln = La, Gd) complexes with 1,10-phenanthroline. Russ J Coord Chem 42, 423–428 (2016). https://doi.org/10.1134/S1070328416070034
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328416070034