Abstract
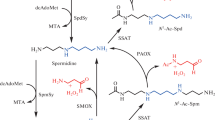
A convenient two-step synthesis of conjugates of HS-CoA and D-pantetheine with aminooxy analogues of Spm and Spd was suggested. The use of acetone linker provided target conjugates with quantitative yields. The activity of CoA-derived “bisubstrate” inhibitors, being active at microM concentrations, was at least 100 times better than that of corresponding derivatives of D-pantetheine.
Similar content being viewed by others
Abbreviations
- CoASH and CoASAc:
-
coenzyme A and its acetyl derivative
- D-Pant:
-
N-(D-pantothenyl)aminoethyl mercaptan
- Put:
-
putrescine (1,4-diaminobutane)
- Spm:
-
spermine (1,12-diamino-4,9-diazadodecane)
- nor-Spd:
-
nor-spermidine (1,7-diamino-4-azaheptane)
- Spd:
-
spermidine (1,8-diamino-4-azaoctane)
- SSAT:
-
spermidine/spermine-N 1-acetyltransferase
References
Wolfenen, R., Bioorg. Med. Chem., 1999, vol. 7, pp. 647–652.
Müller, I.B., Wu, F., Bergmann, B., Knockel, J., Walter, R.D., Gehring, H., and Wrenger, C., PLoS ONE, 2009, vol. 4, p. e4406.
Khomutov, R.M., Dixon, H.B., Vdovina, L.V., Kirpichnikov, M.P., Morozov, Y.V., Severin, E.S., and Khurs, E.N., Biochem. J., 1971, vol. 124, pp. 99–106.
Somu, R.V., Boshoff, H., Qiao, C., Bennett, E.M., Barry, C.E.III., and Aldrich, C.C., J. Med. Chem., 2006, vol. 49, pp. 31–34.
Kappler, F., Vrudhula, V.M., and Hampton, A., J. Med. Chem., 1987, vol. 30, pp. 1599–1603.
Hickman, A.B., Namboodiri, M.A.A., Klein, D.C., and Dyda, F., Cell, 1999, vol. 97, pp. 361–369.
Brown, M.J.B., Mensah, L.M., Doyle, M.L., Broom, N.J.P., Osbourne, N., Forrest, A.K., Richardson, C.M., O’Hanlon, P.J., and Pope, A.J., Biochemistry, 2000, vol. 39, pp. 6003–6011.
Yu, M., Magalhaes, M.L.B., Cook, P.F., and Blanchard, J.S., Biochemistry, 2006, vol. 45, pp. 14788–14794.
Cullis, P.M., Wolfenden, R., Cousens, L.S., and Alberts, B.M., J. Biol. Chem., 1982, vol. 257, pp. 12165–12169.
Ervin, B., Persson, L., and Pegg, A.E., Biochemistry, 1984, vol. 23, pp. 4250–4255.
Hegde, S.S., Chandler, J., Vetting, M.W., Yu, M., and Blanchard, J.S., Biochemistry, 2007, vol. 46, pp. 7187–7195.
Wu, R., Saab, N.H., Hyang, H., Wiest, L., Pegg, A.E., Casero, R.A., and Woster, P.M., Bioorg. Med. Chem., 1996, vol. 4, pp. 825–836.
Libby, P.R. and Porter, C.W., Biochem. Pharmacol., 1992, vol. 44, pp. 830–832.
Fierke, C.A. and Jencks, W.P., J. Biol. Chem., 1986, vol. 261, pp. 7603–7606.
Khomutov, A.R., Vepsalainen, J., Shvetsov, A.S., Hyvonen, T., Keinanen, T.A., Pustobaev, V.N., Eloranta, T.O., and Khomutov, R.M., Tetrahedron, 1996, vol. 52, pp. 13751–13766.
Khomutov, A.R., Svetsov, A.S., Vepsalainen, J.J., and Kritzyn, A.M., Tetrahedron Lett., 2001, vol. 42, pp. 2887–2889.
Simonyan, A.R., Vepsalainen, J., and Khomutov, A.R., Russ. J. Bioorg. Chem, 2006, vol. 32, pp. 578–585.
Casero, R.A. and Marton, L.J., Nat. Rev. Drug Discov., 2007, vol. 6, pp. 373–390.
Seiler, N., Curr. Drug Targets, 2003, vol. 4, pp. 537–564.
Wallace, H.M. and Fraser, A.V., Amino Acids, 2004, vol. 26, pp. 353–365.
Casero, R.A. and Woster, P.M., J. Med. Chem., 2009, vol. 52, pp. 4551–4573.
Roblot, G., Wylde, R., Martin, A., and Parello, J., Tetrahedron, 1993, vol. 29, pp. 6381–6398.
Della, RagioneF. and Pegg, A.E., Biochemistry, 1982, vol. 21, pp. 701–707.
Khomutov, A.R., Biokhimiya, 2002, vol. 67, pp. 1403–1412.
Weisell, J., Hyvonen, M.T., Alhonen, L., Vepsalainen, J., Keinanen, T.A., and Khomutov, A.R., Curr. Pharm. Design, May 16 [Epub ahead of print] (in press).
Ellman, G.L., Arch. Biochem. Biophys., 1959, vol. 82, pp. 70–77.
Bernacki, R.J., Oberman, E.J., Seweryniak, K.E., Atwood, A., Bergeron, R.J., and Porter, C.W., Clin. Cancer Res,, 1995, vol. 1, pp. 847–857.
Cox, B.G. and Hammes, G.G., Proc. Natl., Acad. Sci. U.S.A., 1983, vol. 80, pp. 4233–4237.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.A. Keinanen, T. Hyvonen, J. Vepsalainen, L. Alhonen, A.R. Khomutov, J. Janne, 2014, published in Bioorganicheskaya Khimiya, 2014, Vol. 40, No. 2, pp. 170–177.
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Keinanen, T.A., Hyvonen, T., Vepsalainen, J. et al. Stable analogues of coenzyme-substrate complex of spermidine/spermine-N 1-acetyltransferase reaction. Synthesis and interaction with the enzyme. Russ J Bioorg Chem 40, 155–161 (2014). https://doi.org/10.1134/S1068162014020071
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162014020071