Abstract
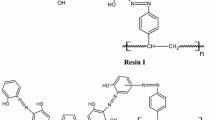
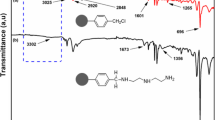
In the present paper, solid-phase extractive preconcentration and separation of lanthanum(III) and cerium(III) using calix[4]arene-o-vanillinsemicarbazone immobilized on a polymeric matrix, a Merrifield peptide resin, is proposed. The diamino derivative of calix[4]arene was first diazotized and coupled with o-vanillinsemicarbazone to obtain a new “upper-rim” functionalized calix[4]arene-o-vanillinsemicarbazone. It was then covalently linked to the Merrifield peptide resin and characterized by FT-IR and elemental analysis. Quantitative studies were carried out by spectrophotometry and ICP-AES with a relative standard deviation of 1.7%. Various physicochemical parameters like pH, concentration of eluting agents, flow rate, total sorption capacity, metal-ligand stoichiometry, exchange kinetics, preconcentration factor, distribution coefficient, breakthrough capacity, resin stability, and effect of electrolytes and associated metal ions have been studied. The uptake and stripping of these metal ions on the resin was fast, indicating a better accessibility of La(III) and Ce(III) towards the chelating sites. Detection limits corresponded to three times the standard deviation of the blank (3σB) and amounted to 3.05 and 6.86 µg/L, along with preconcentration factors of 153 and 133 for La(III) and Ce(III), respectively. The robustness of the procedure is demonstrated by the recoveries obtained (>97.5%) for La(III) and Ce(III) in the presence of several cations and anions. The proposed method was satisfactorily applied to the separation of La(III) and Ce(III) from each other and also from U(VI) and Th(IV) by sequential acidic elution and varying pH. The validity of the method was tested by analyzing these metal ions in monazite sand and standard geological materials.
Similar content being viewed by others
References
Rao, T.P. and Preetha, C.R., Sep. Purif. Rev., 2003, vol. 32, p. 1.
Camel, V., Spectrochim. Acta, 2003, vol. 58, p. 1177.
Zu, X., Liu, P., Pu, Q.S., Sun, Q.Y., and Su, Z.X., Talanta, 2004, vol. 62, p. 918.
Bagheri, H., Gholami, A., and Najafi, A., Anal. Chim. Acta, 2000, vol. 424, p. 233.
Calixarenes Revisited, Monographs in Supramolecular Chemistry, Gutsche, C.D. and Stoddart, J.F., Eds., Cambridge: Royal Society of Chemistry, 1998.
Roundhill, D.M., Prog. Inorg. Chem., 1996, vol. 43, p. 533.
Solution State Metal Complexes of Calixarenes and Polymeric Calixarenes: Handbook of Engineering Polymeric Materials, Yilmaz, M. and Cheremisinoff, N., Eds., New York: Marcel Dekker, 1997, p. 339.
Shinkai, S., Mori, S., Koreishi, H., Tsubaki, T., and Manabe, O., J. Am. Chem. Soc., 1986, vol. 108, p. 2409.
Yilmaz, M. and Deligoz, H., Macromol. Rep., 1994, vol. 31, p. 137.
Deligoz, H. and Yilmaz, M., J. Pol. Sci., Part A: Polym. Chem., 1994, vol. 32, p. 2961.
Deligoz, H. and Yilmaz, M., J. Pol. Sci., Part A: Polym. Chem., 1995, vol. 33, p. 2851.
Deligoz, H. and Yilmaz, M., Solvent, Extr. Ion Exch., 1995, vol. 13, p. 19.
Deligoz, H. and Yilmaz, M., React. Funct. Polym., 1996, vol. 31, p. 81.
Alexandratos, S.D. and Natesan, S., Macromolecules, 2001, vol. 34, p. 206.
Sliwa, W., Croat. Chemica Acta, 2002, vol. 75, p. 131.
Ludwig, R. and Dzung, N.T.K., Sensors, 2002, vol. 2, p. 397.
Ludwig, R., Fresenius’ J. Anal. Chem., 2000, vol. 367, p. 103.
Liang, L., Haese, P.C.D., Lamberts, L.V., van de Vyver, F.L., and De Broe, E., Anal. Chem., 1991, vol. 63, p. 423.
Kramer, K.J.M. and de Haan, E.P.M., van het Groenewoud, H., Dorten, W., Kramer, G.N., Muntau, H., and Quevauviller, P., Trends Anal. Chem., 2002, vol. 21, p. 762.
Garg, B.S. and Jain, V.K., Microchem. J., 1988, vol. 38, p. 144.
Marczenko, Z., Spectrophotometric Determination of Elements, Chichester: Ellis-Horwood, 1976.
Dean, J.A., Lange’s Handbook of Chemistry, 15th ed., New York: McGraw-Hill, 1999.
Gutsche, C.D. and Iqbal, M., Org. Synth. Coll., 1993, vol. 8, p. 75.
Gutsche, C.D. and Lin, L., Tetrahedron, 1986, vol. 42, p. 1633.
Van Loon, J.D., Arduini, A., Coppi, L., Verboom, W., Pochini, A., Ungaro, R., Harkema, S., and Reinhoudt, D.N., J. Org. Chem., 1990, vol. 55, p. 5639.
Kanesato, M., Yokoyama, T., and Suzuki, T.M., Bull. Chem. Soc. Jpn., 1989, vol. 62, p. 3451.
Lajunen, L.H.J., Spectrochemical Analysis by Atomic Absorption and Emission, Cambridge: Royal Society of Chemistry, 1992, p. 9.
Hill, S.J., Inductively Coupled Plasma Spectrometry and Its Applications, Sheffield: Academic Press, 1999.
Author information
Authors and Affiliations
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Jain, V.K., Pandya, R.A., Pillai, S.G. et al. Solid-phase extractive preconcentration and separation of lanthanum(III) and cerium(III) using a polymer-supported chelating calix [4] arene resin. J Anal Chem 62, 104–112 (2007). https://doi.org/10.1134/S1061934807020025
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S1061934807020025