Abstract
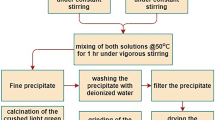
Cerium monoaluminate Ce 3+1-x Ce 4+ x AlO3+x/2 powders with low contents of Ce4+ cations (х ~ 0.052) were synthesized. A set of modern local structure sensitive methods of analysis, including X-ray absorption spectroscopy and Raman spectroscopy, were used to study the crystal, local, and electronic structures of the synthesized compounds. The degree of reduction and the thermal stability to oxidation of reduced powders depend not only on the reduction conditions but also on the conditions of heat pretreatment of the initial samples. It was concluded that the reaction 4CeAlO3 + O2 ↔ 4CeO2 + 2Al2O3 is reversible.
Similar content being viewed by others
References
A. I. Leonov, High-Temperature Chemistry of Cerium Oxide Compounds (Nauka, Leningrad, 1969) [in Russian].
B. A. Arsen’ev, L. S. Kovba, and Kh. S. Bagdasarov, The Chemistry of Rare Elements (Nauka, Moscow, 1983) [in Russian].
Q. Yuan, H.-H. Hao-Hong Duan, L.-L. Li, et al., J. Coll. Inter. Sci, 335, 151 (2009).
V. K. Ivanov, A. B. Shcherbakov, A. E. Baranchikov, et al., Nanocrystalline Ceria: Properties, Synthesis, Application (Izd. Tomsk. Univ., Tomsk, 2013) [in Russian].
V. K. Ivanov, G. P. Kopitsa, A. E. Baranchikov, et al., Russ. J. Inorg. Chem. 54, 1857 (2009).
V. K. Ivanov, A. E. Baranchikov, O. S. Polezhaeva, et al., Russ. J. Inorg. Chem. 55, 325 (2010).
O. V. Safonova, A. A. Guda, C. Paun, et al., J. Phys. Chem. 118, 1974.
E. M. Moroz, Russ. Chem. Rev. 80, 293 (2011).
J. Kašpar, M. Graziani, and P. Fornasiero, Handbook on the Physics and Chemistry of Rare Earths: The Role of Rare Earths in Catalysis, Ed. by K. A. Gschneidner, and L. Eyring (Elsevier Science, Amsterdam, 2000), Vol. 29, p. 159.
S. A. Venâncio and P. E. V. de Miranda, Ceram. Int. 37, 3139 (2011).
A. I. Mikhailichenko, E. B. Mikhlin, and Yu. B. Patrikeev, Rare-Earth Metals (Metallurgiya, Moscow, 1987) [in Russian].
P. Maestro and D. Huguenin, J. Alloys Compd. 225, 520 (1995).
A. Feteira, D. C. Sinclair, and M. T. Lanagan, J. Appl. Phys. 101, 064110 (2007).
T. Shishido, S. Okada, Kudou K. Kunio, et al., Pacific Sci. Rev. 10, 45 (2008).
L. Vasylechko, A. Senyshyn, D. Trots, et al., J. Solid State Chem. 180, 1277 (2007).
G. R. Rao and B. G. Mishra, Bull. Catal. Soc. Ind. 2, 122 (2003).
P. A. Deshpande, S. T. Aruna, and G. Madras, Catal. Sci. Technol. 1, 1683 (2011).
P. A. Deshpande, S. T. Aruna, and G. Madras, Clean Soil, Air, Water 39, 259 (2011).
V. V. Popov, Ya. V. Zubavichus, A. P. Menushenkov, et al., Russ. J. Inorg. Chem. 60, 16 (2015).
A. P. Hammersley, S. O. Svensson, M. Hanfland, et al., High Press. Res. 14, 235 (1996).
V. Petricek, M. Dusek, and L. Palatinus, Jana 2006, The Crystallographic Computing System, Praha, Czech. Republic, Institute of Physics, 2006.
K. V. Klementiev, http://www.cells.es/old/Beamlines/CLAESS/software/xanda.html.
B. M. Reddy, A. Khan, P. Lakshmanan, et al., J. Phys. Chem. B 109, 3355 (1979).
G. Gouadec and Ph. Colomban, Prog. Cryst. Growth Charact. Mater. 53, 1 (2007).
I. R. Lewis and H. G. V. Edwards, Handbook of Raman Spectroscopy (Marcel Dekker, New York, 2001).
A. V. Soldatov, T. S. Ivanchenko, S. Della Longa, et al., Phys. Rev. B 50, 5074 (1994).
V. Fernandes, I. L. Graff, J. Varalda, et al., J. Electrochem. Soc. 159, 27 (2012).
D. I. Khomskii, Usp. Fiz. Nauk 129, 443 (1979).
P. E. R. Blanchard, S. Liu, D. J. Kennedy, et al., J. Phys. Chem. 117, 2266 (1979).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Popov, A.P. Menushenkov, Ya.V. Zubavichus, A.S. Sharapov, V.A. Kabanova, A.A. Yastrebtsev, L.A. Arzhatkina, N.A. Tsarenko, A.M. Strel’nikova, V.V. Kurilkin, 2016, published in Zhurnal Neorganicheskoi Khimii, 2016, Vol. 61, No. 2, pp. 238–244.
Rights and permissions
About this article
Cite this article
Popov, V.V., Menushenkov, A.P., Zubavichus, Y.V. et al. Effect of the synthesis conditions on the crystal, local, and electronic structure of Ce 3+1-x Ce 4+ x AlO3 + x/2 . Russ. J. Inorg. Chem. 61, 225–231 (2016). https://doi.org/10.1134/S0036023616020170
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023616020170