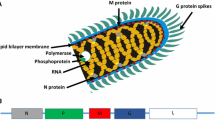
Abstract
Rabies is an infectious disease among humans and animals that remains incurable, despite its longstanding research history. The only way to prevent the disease is prompt treatment, including vaccination as an obligatory component and administration of antirabies immunoglobulin as a supplement. Since the first antirabies vaccination performed in the 19th century, a large number of different rabies vaccines have been developed. Progress in molecular biology and biotechnology enabled the development of effective and safe technologies of vaccine production. Currently, new-generation vaccines are being developed based on recombinant rabies virus strains or on the production of an individual recombinant rabies antigen—glycoprotein (G protein), either as a component of nonpathogenic viruses, or in plants, or in the form of DNA vaccines. In this review, the main modern trends in the development of rabies vaccines have been discussed.
Similar content being viewed by others
Abbreviations
- WHO:
-
World Health Organization
- CRV:
-
culturebased rabies vaccine
References
http://www.who.int/mediacentre/factsheets/fs099/ru/
http://www.rg.ru/2012/03/23/beshenstvo-dok.html
Rupprecht C.E., Plotkin S.A. 2012. Rabies vaccines. In: Vaccines, 6th ed., vol.2. Eds. Plotkin S.A., Orenstein W.A., Offit P.A. Scotland: Elsevier, pp. 646–668.
Culbertson C.G., Peck F.B., Jr., Powell H.M. 1956. Duck-embryo rabies vaccine: Study of fixed virus vaccine grown in embryonated duck eggs and killed with beta-propiolactone (BPL). J. Am. Med. Assoc. 162, 1373–1376.
Kissling R.E. 1958. Growth of rabies virus in non-nervous tissue culture. Proc. Soc. Exp. Biol. Med. 98, 223–225.
Fenje P. 1960. A rabies vaccine from hamster kidney tissue cultures: Preparation and evaluation in animals. Can. J. Microbiol. 6, 605–609.
http://www.who.int/rabies/931/en/
Briggs D.J., Nagarajan T., Rupprecht C.E. 2013. Rabies vaccines. In: Rabies: Scientific Basis of the Disease and Its Management, 3rd ed., vol. 13. Ed. Jackson A.C. Oxford: Elsevier, pp. 497–526.
http://www.who.int/immunization/documents/WHO_ pp_rabies_2010_RU.pdf
Movsesyants A.A., Ageenko G.B. 2006. Anti-rabies agents used in the Russian Federation. RET Info. 1, 44–45. https://docs.google.com/document/d/1RpvtqmQbmHc2kK2DEDcD4MBCMQ73gci9ZEhjgg9q-A/edit?pli=10
Mukhacheva A.V., Movsesyants A.A., Alsynbaev M.M. 2014. Selection of optimal methods for purifying protein substances contained in cultural concentrated purified inactivated antirabies vaccine (KOKAV). Epidemiol. Vaktsinoprofilakt. 3, 84–88.
Gribencha S.V., Losin M.A., Gribencha L.F., Nepoklonova I.V. 2012. A new principle of vaccinal virus selection based on the expression level of G protein, the main rabies virus immunogen. Vopr. Virusol. 57, 44–48.
Pukhova N.M., Samuilenko A.Ya., Elakov A.L. 2012. Reference preparation for controlling immunogenicity of animal rabies vaccines. Vet. Vrach. 5–7.
Losich M.A., Nepoklonova I.V., Mukhin A.N., Raev S.A., Seliverstov A.S., Gribencha S.V., Verkhovskii O.A., Aliper T.I. 2012. Revelopment and immunological properties of Rabifel, a new antirabies vaccine. Ross. Vet. Zh. Melkie Domash. Dikie Zhivotn. 10–14.
Avdeeva Zh.I., Alpatova N.A., Akol’zina S.E., Medunitsyn N.V. 2009. Immunoadjuvant effect of cytokines. Tikhook, Med. Zh. 19–22.
Liu X., Yang Y., Sun Z., Chen J., Ai J., Dun C., Fu Z.F., Niu X., Guo X. 2014. A recombinant rabies virus encoding two copies of the glycoprotein gene confers protection in dogs against a virulent challenge. PLOS ONE. 9, e87105.
Tao L., Ge J., Wang X., Wen Z., Zhai H., Hua T., Zhao B., Kong D., Yang C., Bu Z. 2011. Generation of a recombinant rabies Flury LEP virus carrying an additional G gene creates an improved seed virus for inactivated vaccine production. Virol. J. 8, 454.
Tuffereau C., Leblois H., Benejean J., Coulon P., Lafay F., Flamand A. 1989. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology. 172, 206–212.
Faber M., Faber M.L., Papaneri A., Bette M., Weihe E., Dietzschold B., Schnell M.J. 2005. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J. Virol. 79, 14141–14148.
Dietzschold M.L., Faber M., Mattis J.A., Pak K.Y., Schnell M.J., Dietzschold B. 2004. In vitro growth and stability of recombinant rabies viruses designed for vaccination of wildlife. Vaccine. 23, 518–524.
Faber M., Li J., Kean R.B., Hooper D.C., Alugupalli K.R., Dietzschold B. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc. Natl. Acad. Sci. U. S. A. 106, 11300–11305.
Schutsky K., Curtis D., Bongiorno E.K., Barkhouse D.A., Kean R.B., Dietzschold B., Hooper D.C., Faber M. 2013. Intramuscular inoculation of mice with the liveattenuated recombinant rabies virus TriGAS results in a transient infection of the draining lymph nodes and a robust, long-lasting protective immune response against rabies. J. Virol. 87, 1834–1841.
Norton J.E. Jr., Lytle A.G., Shen S., Tzvetkov E.P., Dorfmeier C.L., Mc Gettigan J.P. 2014. ICAM-1-based rabies virus vaccine shows increased infection and activation of primary murine B cells in vitro and enhanced antibody titers in-vivo. PLOS ONE. 9, e87098.
Wiktor T.J., Macfarlan R.I., Reagan K.J., Dietzschold B., Curtis P.J., Wunner W.H., Kieny M.P., Lathe R., Lecocq J.P., Mackett M., et al. 1984. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc. Natl. Acad. Sci. U. S. A. 81, 7194–7198.
Cliquet F., Barrat J., Guiot A.L., Cael N., Boutrand S., Maki J., Schumacher C.L. 2008. Efficacy and bait acceptance of vaccinia vectored rabies glycoprotein vaccine in captive foxes (Vulpes vulpes), raccoon dogs (Nyctereutes procyonoides), and dogs (Canis familiaris). Vaccine. 26, 4627–4638.
Weyer J., Rupprecht C.E., Nel L.H. 2009. Poxvirusvectored vaccines for rabies: A review. Vaccine. 27, 7198–7201.
Poulet H., Minke J., Pardo M.C., Juillard V., Nordgren B., Audonnet J.C. 2007. Development and registration of recombinant veterinary vaccines. The example of the canarypox vector platform. Vaccine. 25, 5606–5612.
Amann R., Rohde J., Wulle U., Conlee D., Raue R., Martinon O., Rziha H.J. 2013. A new rabies vaccine based on a recombinant ORF virus (parapoxvirus) expressing the rabies virus glycoprotein. J. Virol. 87, 1618–1630.
Yarosh O.K., Wandeler A.I., Graham F.L., Campbell J.B., Prevec L. 1996. Human adenovirus type 5 vectors expressing rabies glycoprotein. Vaccine. 14, 1257–1264.
Tims T., Briggs D.J., Davis R.D., Moore S.M., Xiang Z., Ertl H.C., Fu Z.F. 2000. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine. 18, 2804–2807.
Xiang Z.Q., Gao G.P., Reyes-Sandoval A., Li Y., Wilson J.M., Ertl H.C. 2003. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 77, 10780–10789.
Shen C.F., Lanthier S., Jacob D., Montes J., Beath A., Beresford A., Kamen A. 2012. Process optimization and scale-up for production of rabies vaccine live adenovirus vector (AdRG1.3). Vaccine. 30, 300–306.
Fehlner-Gardiner C., Rudd R., Donovan D., Slate D., Kempf L., Badcock J. 2012. Comparing ONRAB and RABORAL V-RG® oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA J. Wild. Dis. 48, 157–167.
Yang D.K., Kim H.H., Lee K.W., Song J.Y. 2013. The present and future of rabies vaccine in animals. Clin. Exp. Vaccine Res. 2, 19–25.
Liu Y., Zhang S., Ma G., Zhang F., Hu R. 2008. Efficacy and safety of a live canine adenovirus-vectored rabies virus vaccine in swine. Vaccine. 26, 5368–5372.
Chen Z., Zhou M., Gao X., Zhang G., Ren G., Gnanadurai C.W., Fu Z.F., He B. 2013. A novel rabies vaccine based on a recombinant parainfluenza virus 5 expressing rabies virus glycoprotein. J. Virol. 87, 2986–2993.
Mc Garvey P.B., Hammond J., Dienelt M.M., Hooper D.C., Fu Z.F., Dietzschold B., Koprowski H., Michaels F.H. 1995. Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology (NY). 13, 1484–1487.
Ashraf S., Singh P.K., Yadav D.K., Shahnawaz M., Mishra S., Sawant S.V., Tuli R. 2005. High level expression of surface glycoprotein of rabies virus in tobacco leaves and its immunoprotective activity in mice. J. Biotechnol. 119, 1–14.
Yusibov V., Hooper D.C., Spitsin S.V., Fleysh N., Kean R.B., Mikheeva T., Deka D., Karasev A., Cox S., Randall J., et al. 2002. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine. 20, 3155–3164.
Rojas-Anaya E., Loza-Rubio E., Olivera-Flores M.T., Gomez-Lim M. 2009. Expression of rabies virus G protein in carrots (Daucus carota). Transgenic Res. 18, 911–919.
Loza-Rubio E., Rojas E., Gomez L., Olivera M.T., Gomez-Lim M.A. 2008. Development of an edible rabies vaccine in maize using the Vnukovo strain. Dev. Biol. (Basel). 131, 477–482.
Loza-Rubio E., Rojas-Anaya E., Lopez J., Olivera Flores M.T., Gomez-Lim M., Tapia-Perez G. 2012. Induction of a protective immune response to rabies virus in sheep after oral immunization with transgenic maize, expressing the rabies virus glycoprotein. Vaccine. 30, 5551–5556.
Graham B.S. 2013. Advances in antiviral vaccine development. Immunol. Rev. 255, 230–242.
Kutzler M.A., Weiner D.B. 2008. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 9, 776–788.
Redding L., Weiner D.B. 2009. DNA vaccines in veterinary use. Expert Rev. Vaccines. 8, 1251–1276.
Li L., Saade F., Petrovsky N. 2012. The future of human DNA vaccines. J. Biotechnol. 162, 171–182.
Villarreal D.O., Talbott K.T., Choo D.K., Shedlock D.J., Weiner D.B. 2013. Synthetic DNA vaccine strategies against persistent viral infections. Expert Rev. Vaccines. 12, 537–554.
Kaur M., Garg R., Singh S., Bhatnagar R. 2014. Rabies vaccines: Where do we stand, where are we heading? Exp. Rev. Vaccines. 1–13.
Tuchkov I.V., Nikiforov A.K. 2010. DNA immunization against rabies. Probl. Osobo Opasn. Infekts. 104, 74–78.
Ferraro B., Morrow M.P., Hutnick N.A., Shin T.H., Lucke C.E., Weiner D.B. 2011. Clinical applications of DNA vaccines: Current progress. Clin. Infect. Dis. 53, 296–302.
Hutnick N.A., Myles D.J., Bian C.B., Muthumani K., Weiner D.B. 2011. Selected approaches for increasing HIV DNA vaccine immunogenicity in vivo. Curr. Opin. Virol. 1, 233–240.
Saade F., Petrovsky N. 2012. Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines. 11, 189–209.
Ullas P.T., Desai A., Madhusudana S.N. 2012. Rabies DNA vaccines: Current status and future. World J. Vaccines. 2, 36–45.
Osinubi M.O., Wu X., Franka R., Niezgoda M., Nok A.J., Ogunkoya A.B., Rupprecht C.E. 2009. Enhancing comparative rabies DNA vaccine effectiveness through glycoprotein gene modifications. Vaccine. 27, 7214–7218.
Tesoro Cruz E., Hernandez Gonzalez R., Alonso Morales R., Aguilar-Setien A. 2006. Rabies DNA vaccination by the intranasal route in dogs. Dev. Biol. (Basel). 125, 221–231.
Bahloul C., Taieb D., Diouani M.F., Ahmed S.B., Chtourou Y., B’ Chir B I., Kharmachi H., Dellagi K. 2006. Field trials of a very potent rabies DNA vaccine which induced long lasting virus neutralizing antibodies and protection in dogs in experimental conditions. Vaccine. 24, 1063–1072.
Kaur M., Saxena A., Rai A., Bhatnagar R. 2010. Rabies DNA vaccine encoding lysosome-targeted glycoprotein supplemented with Emulsigen-D confers complete protection in preexposure and postexposure studies in BALB/c mice. FASEB J. 24, 173–183.
Stab V., Nitsche S., Niezold T., Storcksdieck Genannt Bonsmann M., Wiechers A., Tippler B., Hannaman D., Ehrhardt C., Uberla K., Grunwald T., Tenbusch M. 2013. Protective efficacy and immunogenicity of a combinatory DNA vaccine against influenza A virus and the respiratory syncytial virus. PLOS ONE. 8, e72217.
Touihri L., Ahmed S.B., Chtourou Y., Daoud R., Bahloul C. 2012. Design of different strategies of multivalent DNA-based vaccination against rabies and canine distemper in mice and dogs. Virol. J. 9, 319.
Yan J., Corbitt N., Pankhong P., Shin T., Khan A., Sardesai N.Y., Weiner D.B. 2011. Immunogenicity of a novel engineered HIV-1 clade C synthetic consensusbased envelope DNA vaccine. Vaccine. 29, 7173–7181.
Obeng-Adjei N., Hutnick N.A., Yan J., Chu J.S., Myles D.J., Morrow M.P., Sardesai N.Y., Weiner D.B. 2013. DNA vaccine cocktail expressing genotype A and C HBV surface and consensus core antigens generates robust cytotoxic and antibody responses in mice and rhesus macaques. Cancer Gene Ther. 20, 652–662.
Yan J., Villarreal D.O., Racine T., Chu J.S., Walters J.N., Morrow M.P., Khan A.S., Sardesai N.Y., Kim J.J., Kobinger G.P., Weiner D.B. 2014. Protective immunity to H7N9 influenza viruses elicited by synthetic DNA vaccine. Vaccine. 32, 2833–2842.
Latimer B., Toporovski R., Yan J., Pankhong P., Morrow M.P., Khan A.S., Sardesai N.Y., Welles S.L., Jacobson J.M., Weiner D.B., Kutzler M.A. 2014. Strong HCV NS3/4a, NS4b, NS5a, NS5b-specific cellular immune responses induced in rhesus macaques by a novel HCV genotype 1a/1b consensus DNA vaccine. Hum. Vaccin. Immunother. 10, 2357–2365.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.S. Starodubova, O.V. Preobrazhenskaia, Y.V. Kuzmenko, A.A. Latanova, E.I. Yarygina, V.L. Karpov, 2015, published in Molekulyarnaya Biologiya, 2015, Vol. 49, No. 4, pp. 577–584.
Rights and permissions
About this article
Cite this article
Starodubova, E.S., Preobrazhenskaia, O.V., Kuzmenko, Y.V. et al. Rabies vaccines: Current status and prospects for development. Mol Biol 49, 513–519 (2015). https://doi.org/10.1134/S0026893315040172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893315040172