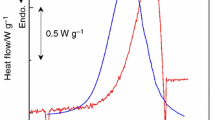
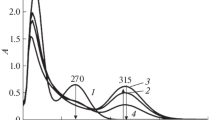
Abstract
The kinetics of the decomposition of thiourea dioxide in aqueous ammonia solution at various temperatures and ammonia concentrations has been investigated. The parameters of the individual steps of the process have been derived from experimental data by solving an inverse kinetic problem. The adequacy of the proposed kinetic model to the experimental data has been verified on the basis of the F-test and calculated correlation coefficients for the rate constants of the individual steps of the process.
Similar content being viewed by others
References
Makarov, S.V., Russ. Chem. Rev., 2001, vol.70, no. 10, p. 885.
Polenov, Yu.V., Makarov, S.V., and Budanov, V.V., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 1986, vol.29, no. 12, p. 30.
Svarovsky, S.A., Simoiy, R.H., and Makarov, S.V., J. Chem. Soc., Dalton Trans., 2000, p. 511.
Polenov, Yu.V., Egorova, E.V., and Makarova, E.V., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2013, vol.56, no. 10, p. 75.
Budanov, V.V., Khimiya i tekhnologiya vosstanovitelei na osnove sul’foksilovoi kisloty: Rongalit i ego analogi (Chemistry and Technology of Reductants Based on Sulfoxylic Acid: Rongalite and Its Analogues), Moscow: Khimiya, 1984.
Shafran, I.G., Stepanova, A.G., and Pankrat’eva, L.I., Trudy IREA: Khim. Reakt. Prep., 1963, vol.25, p. 215.
Ermolina, S.V., Makarov, S.V., Terskaya, I.N., and Budanov, V.V., Zh. Neorg. Khim., 1995, vol.40, no. 9, p. 1466.
Rath, H. and Rau, J., Melliand Textilber, 1954, vol. 35, no. 10, p. 1125.
Kunin, T.I., Zh. Prikl. Khim., 1949, vol.22, no. 2, p. 199.
Polenov, Yu.V., Pushkina, V.A., and Egorova, E.V., Russ. J. Gen. Chem., 2001, vol.71, no. 5, p. 675.
Spravochnik khimika (Chemist’s Handbook), Leningrad: Khimiya, 1964, vol. 3, p. 316.
Polenov, Yu.V., Pushkina, V.A., Egorova, E.V., Labutin, A.N., and Khalizov, R.A., Kinet. Catal., 2002, vol.43, no. 4, p. 465.
Gao, Q., Liu, B., Li, L., and Wang, J., J. Phys. Chem. A, 2007, vol. 111, no. 5, p. 872.
Gartman, T.N. and Klushin, D.V., Osnovy komp’yuternogo modelirovaniya khimiko-tekhnologicheskikh protsessov (Computer Simulation of Processes in Chemical Engineering), Moscow: Akademkniga, 2006.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.V. Polenov, E.V. Makarova, E.V. Egorova, 2014, published in Kinetika i Kataliz, 2014, Vol. 55, No. 5, pp. 594–598.
Rights and permissions
About this article
Cite this article
Polenov, Y.V., Makarova, E.V. & Egorova, E.V. Kinetic model of thiourea dioxide decomposition in aqueous ammonia. Kinet Catal 55, 566–570 (2014). https://doi.org/10.1134/S0023158414040120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158414040120