Abstract
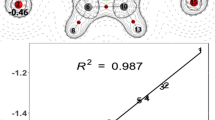
The geometries of ten benzenoid energetic materials are fully optimized by employing B3LYP and B3P86 methods with the 6–31G** basis set. Bond dissociation energies (BDEs) for the removal of the NO2 group in benzenoid molecules are calculated at the same level. The calculation results show that the insertion of an electron withdrawing group increases the stability of the molecules, while the insertion of an electron donating group reduces the stability of the molecules. In addition, the relationship between the impact sensitivities and the weakest BDE values is examined. There exists a good linear correlation between the impact sensitivity and the ratio of the BDE value to the molecular total energy.
Similar content being viewed by others
References
D. H. Andrews, Phys. Rev., 36, 544–554 (1990).
E. V. Holtz, D. O. Ornellas, and M. F. Foltz, Propellants, Explosives, Pyrotechnics, 19, 206–212 (1994).
M. F. Foltz, Propellants, Explosives, Pyrotechnics, 19, 63–96 (1994).
A. K. Sikder, and N. Sikder, J. Hazard. Mater., 112, 1–15 (2004).
A. K. Sikder, G. Maddalla, J. P. Agraval, and H. Singh, J. Hazard. Mater., 84, 1–26 (2001).
L. E. Fried, M. R. Manaa, P. F. Pagoria, and R. L. Simpson, Ann. Rev. Mater. Res., 31, 291–323 (2001).
M. J. Kamlet and H. G. Adolph, Propellants, Explosives, Pyrotechnics, 4, 30–34 (1979).
M. J. Kamlet and H. G. Adolph, Proc. 7th Symp. Deton., Report No. NSWCMP-82-334, Naval SurfaceWarfare Center, Silver Springs, MD (1981), p. 84.
M. J. Kamlet, Proc. 6th Symp. Deton., Report No. ACR 221, Office of Naval Research (1976), p. 312.
F. J. Owens, J. Mol. Struct. (Theochem.), 370, 11–16 (1996).
T. B. Brill and K. J. James, Chem. Rev., 93, 2667–2692 (1993).
P. Politzer and J. S. Murray, J. Mol. Struct., 376, 419–424 (1996).
B. M. Rice, S. Sahu, and F. J. Owens, J. Mol. Struct. (Theochem.), 583, 69–72 (2002).
X. H. Li, Z. X. Tang, and X. Z. Zhang, J. Hazard. Mater., 183, 622–631 (2010).
R. W. Hakala, Intern. J. Quant. Chem., 1, 187–196 (1967).
R. Meyer, J. Kohler, and A. Homburg, Explosives, Wiley-VCH, Weinheim (2002).
X. H. Li, R. Z. Zhang, X. Z. Zhang, X. D. Yang, and X. L. Cheng, Chinese J. Struct. Chem., 26, 1481–1485 (2007).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, and J. A. Pople, Gaussian 03, Revision B.02, Gaussian Inc., Pittsburgh, PA (2003).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–789 (1988).
J. P. Perdew, Phys. Rev. B, 33, 8822–8824 (1986).
P. C. Chen, Y. C. Chieh, and S. C. Tzeng, J. Mol. Struct. (Theochem.), 634, 215–224 (2003).
X. H. Li, Z. X. Tang, X. Z. Zhang, and X. D. Yang, J. Hazard. Mater., 165, 372–378 (2009).
B. M. Rice, S. Sahu, and F. J. Owens, J. Mol. Struct.(Theochem.), 583, 69–72 (2002).
S. W. Benson, Thermochemical Kinetics, 2d ed., Wiley-Interscience, New York (1976).
M. J. Kamlet, Proceeding 6 Intern. Symp. Detonation, CA, San Diego (1976).
G. S. Chung, M. W. Schimidt, and M. S. Gordon, J. Phys. Chem. A, 104, 5647–5650 (2000).
X. H. Li, Z. X. Tang, Abraham F. Jalbout, X. Z. Zhang, and X. L. Cheng, J. Mol. Struct. (Theochem.), 854, 76 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2013 by X.-H. Li, D.-F. Han, X.-Z. Zhang
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 54, No. 3, pp. 447–452, May–June, 2013.
Rights and permissions
About this article
Cite this article
Li, X.H., Han, D.F. & Zhang, X.Z. Investigation of correlation between impact sensitivities and bond dissociation energies in benzenoid nitro compounds. J Struct Chem 54, 499–504 (2013). https://doi.org/10.1134/S0022476613030049
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476613030049