Abstract
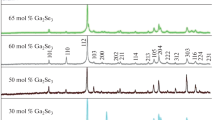
The Ho2S3-Ga2S3 system has been studied using differential thermal analysis, X-ray diffraction, microstructural analysis, microhardness tests, and density measurements, and its phase diagram has been constructed. The system contains three ternary compounds: Ho3GaS6, HoGaS3, and Ho6Ga10/3S14. Their melting behavior has been studied for the first time. The compound Ho6Ga10/3S14 melts congruently at 1435 K; Ho3GaS6 and HoGaS3 melt incongruently at 1370 and 1250 K, respectively. The Ho2S3-Ga2S3 system is a pseudobinary join of the ternary system Ho-Ga-S. At room temperature, the β-Ga2S3-based solid solution extends to 1.5 mol % Ho2S3; the Ho2S3 solubility in γ-Ga2S3 is 10 mol %. The compounds HoGaS3 and Ho3GaS6 crystallize in orthorhombic symmetry (Ho3GaS6: a = 10.40 Å, b = 13.20 Å, c = 6.44 Å, Z = 4; HoGaS3: a = 6.8 Å, b = 9.92 Å, a = 3.08 Å, Z = 4). Ho6Ga10/3S14 has a hexagonal structure (a = 9.62 Å, c = 6.04 Å).
Similar content being viewed by others
References
Medvedeva Z.S. Khal’kogenidy elementov III B gruppy periodicheskoi sistemy (Group IIIB Chalcogenides) Moscow: Nauka, 1968.
Rustamov, P.G., Khal’kogenidy galliya (Gallium Chalcogenides), Baku: Akad. Nauk Az. SSR, 1968.
Samsonov, G.V. and Drozdova, S.V., Sul’fidy (Sulfides), Moscow: Metallurgiya, 1972.
Rustamov, P.G. and Mardakhaev, B.N., Processes for the Synthesis of Sulfur-Containing Alloys and Compounds, Dokl. Akad. Nauk Az. SSR, 1972, vol. 20, no. 9, pp. 13–15.
Aliev, O.M., Rustamov, P.G., Zul’fugarly, J.I., and Agaev, A.B., Group IIIA and IIIB Chalcogenide Systems, Azerb. Khim. Zh., 1980, no. 3, pp. 137–142.
Agaev, A.B., Akhmedova, Dzh.A., and Rustamov, P.G., Ho2S3-In2S3 System, Zh. Neorg. Khim., 1992, vol. 37,issue 2, pp. 461–464.
Flahaunt, J., Handbook on the Physics and Chemistry of Rare Earths.
Lyakishev N.P. Diagrammy sostoyaniya dvoinykh metallicheskikh sistem (Phase Diagrams of Binary Metallic Systems), Moscow: Mashinostroenie, 1997.
Sleight, F.W. and Prewitt, C.T., Inorg. Chem., 1968, vol. 7, no. 11, pp. 2282–2286.
Webb, A.W. and Hall, H.T., Inorg. Chem., 1970, no. 9, pp. 1034–1037.
Eatough, N.L., Webb, A.W., and Hall, H.T., Inorg. Chem., 1969, no. 8, pp. 2069–2072.
Parthe, E., Kristallochimie des structures tetraedriques, Paris: Gordon & Breach, 1972, pp. 295–299.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © C.A. Veliev, 2010, published in Neorganicheskie Materialy, 2010, Vol. 46, No. 5, pp. 520–523.
Rights and permissions
About this article
Cite this article
Veliev, C.A. Ho2S3-Ga2S3 phase diagram. Inorg Mater 46, 452–455 (2010). https://doi.org/10.1134/S002016851005002X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016851005002X