Abstract
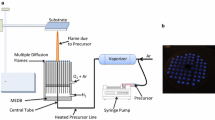
With ferrocene as a precursor, carbon-encapsulated iron nanoparticles are synthesized through detonation of a gas mixture of hydrogen and air in a titanium detonation tube. XRD and TEM characterization shows that a downward trend in the size of particles can be observed with increasing amounts of the precursor. However, no further decrease occurs when the size of nanoparticles reaches approximately ≈40 nm, after which they remain in the range of 30–50 nm. The initial temperature of the detonation tube at 353 K is the optimal initial temperature for the synthesis. The average grain size of the synthesized products becomes larger as the temperature of detonation increases.
Similar content being viewed by others
References
B. B. Bokhonov, “Permeability of Carbon Shells During Sulfidation of Encapsulated Silver Nanoparticles,” Carbon 67, 572–577 (2014).
B. B. Bokhonov and S. A. Novopashin, “In situ Investigation of Morphological and Phase Changes During Thermal Annealing and Oxidation of Carbon- Encapsulated Copper Nanoparticles,” J. Nanopart. Res. 12 (8), 2771–2777 (2010).
P. G. Collins, A. Zettl, H. Bando, A. Thess, and R. E. Smalley, “Nanotube Nanodevice,” Science 278 (3), 100–103 (1997).
K. Bubke, H. Gnewuch, M. Hempstead, J. Hammer, and M. L. H. Green, “Optical Anisotropy of Dispersed Carbon Nanotubes Induced by an Electric Field,” Appl. Phys. Lett. 71 (14), 1906–1908 (1997).
Z. D. Zhang, J. G. Zheng, I. Skorvanek, et al., “Shell/Core Structure and Magnetic Properties of Carbon Coated FeCo(C) Nanocapsules,” J. Phys. Condensed Matter. 13 (9), 1921–1929 (2001).
Z. D. Zhang, J. L. Yu, J. G. Zheng, et al., “Structure and Magnetic Properties of Boron–Oxide–Coated Fe(B) Nanocapsules Prepared by Arc Discharge in Diborane,” Phys. Rev. B 64 (2), 024404(1–5) (2001).
Z. H. Wang, Z. D. Zhang, C. J. Choi, et al., “Structure and Magnetic Properties of Fe(C) and Co(C) Nanocapsules Prepared by Chemical Vapor Condensation,” J. Alloy Compounds 361 (1/2), 289–293 (2003).
X. L. Dong, Z. D. Zhang, S. R. Jin, et al., “Characterization of Fe–Ni(C) Nanocapsules Synthesized by Arc Discharge in Methane,” J. Mater. Res. 14 (5), 1782–1790 (1999).
Y. Yosida, S. Shida, and T. Ohsuna, “Synthesis, Identification, and Growth Mechanism of Fe, Ni, and Co Crystals Encapsulated in Multiwalled Carbon Nanocages,” J. Appl. Phys. 76 (8), 4533–4539 (1994).
J. J. Host, J. A. Block, K. Parvin, et al., “Effect of Annealing on the Structure and Magnetic Properties of Graphite Encapsulated Nickel and Cobalt Nanocrystals,” J. Appl. Phys. 83 (2), 793–801 (1998).
Z. Y. Zhong, H. Y. Chen, S. B. Tang, et al., “Catalytic Growth of Carbon Nanoballs with and without Cobalt Encapsulation,” Chem. Phys. Lett. 330 (1/2), 41–47 (2000).
N. Sano, H. Akazawa, T. Kikuchi, et al., “Separated Synthesis of Iron-Included Carbon Nanocapsules and Nanotubes by Pyrolysis of Ferrocene in Pure Hydrogen,” Carbon 41 (11), 2159–2179 (2003).
P. J. F. Harris and S. C. Tsang, “A Simple Technique for the Synthesis of Filled Carbon Nanoparticles,” Chem. Phys. Lett. 293 (1/2), 53–58 (1988).
H. M. Zhang, Y. Cui, and H. J. Zhong, “Study on Preparation of Amorphous ZnO Nanopowder by Low Temperature Pyrolysis Method,” J. Synth. Cryst. 33 (6), 978–981 (2004).
T. Oyama and K. Takeuchi, “Gas-Phase Synthesis of Crystalline B4C Encapsulated in Graphitic Particles by Pulsed-Laser Irradiation,” Carbon 37 (3), 433–436 (1999).
W. Z. Wu, Z. P. Zhu, Z. Y. Liu, et al., “Preparation of Carbon-Encapsulated Iron Carbide Nanoparticles by an Explosion Method,” Carbon 41 (2), 317–321 (2003).
H. T. Yuan, Y. Feng, L. J. Qiao, et al., “Impact on Performance of Ti, Zr Magnesium-Based Alloys by Graphite Coating,” Power Technol. 28 (4), 216–219 (2008).
X. J. Li, N. Luo, X. Ouyang, et al., “Research Status on Synthesis of Carbon Encapsulated Metal Nanomaterial,” Mater. Rev. 23 (7), 33–37 (2009).
C. X. Lv, Industrial Explosives Theory (Ordnance Industry Press, Beijing, 2003).
B. P. Zhang, Q. M. Zhang, and F. L. Huang, Detonation Physics (Ordnance Industry Press, Beijing, 2009).
H. L. Cao and W. Q. Xu, “The Teaching Discussion of the Observation Experiment of Iron–Carbon Alloy Balance Organization,” Exp. Technol. Management 26 (8), 134–136 (2009).
D. S. Dai and K. M. Qian, Ferromagnetic Science (Science Press, Beijing, 1998), Vol. I.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © H. Yan, T. Zhao, X. Li, Ch. Hun.
Published in Fizika Goreniya i Vzryva, Vol. 51, No. 4, pp. 109–115, July–August, 2015.
Rights and permissions
About this article
Cite this article
Yan, H., Zhao, T., Li, X. et al. Hydrogen and air detonation (deflagration) synthesis of carbon-encapsulated iron nanoparticles. Combust Explos Shock Waves 51, 495–501 (2015). https://doi.org/10.1134/S0010508215040152
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0010508215040152