Abstract
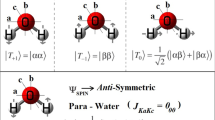
According to the latest results obtained by small-angle X-ray scattering and X-ray spectroscopy, it was suggested that water on a nanometer scale represents a fluctuating mixture of clusters with tetrahedral structure and a subphase with partially broken hydrogen bonds, whereas the nuclear configuration of the H2O molecule corresponds to single tetrahedral coordination. The basic reason of such structural partition is not clear until now. Here we show that it can be associated with existence of two nuclear H2O spin isomers that have different probability to be in one or the other subphase. The para molecule can transfer an excess of its rotational energy to the environment, up to complete stopping of rotation because its rotational quantum number J = 0 in the basic state. This property is favorable for formation of clusters with closed H-bonds. Ortho molecules with odd-numbered J states lack this property and thus should be predominantly present in the locations with impaired bonds.
Similar content being viewed by others
References
A. Farcas, Orthohydrogen, Parahydrogen and Heavy Hydrogen (Cambridge Univ. Press, Cambridge, 1935).
S. Meiboom, J. Chem. Phys. 34, 375 (1961).
V. I. Tikhonov and A. A. Volkov, Science 296, 2363 (2002).
V. G. Artemov, Candidate’s Dissertation (IGP AS, 2010).
P. O. Kapralov, Candidate’s Dissertation (IGP AS, 2010).
S. A. Potekhin and R. S. Khusainova, Biophys. Chem. 118, 84 (2005).
S. M. Pershin, Phys. Wave Phenom. 13, 192 (2005).
S. M. Pershin, Laser Physics 16, 1184 (2006).
A. F. Bunkin and S. M. Pershin, Phys. Wave Phenom. 18, 237 (2010).
S. I. Veber, S. L. Bagryanskaya, and P. L. Chapovskii, Zh. Eksp. Teor. Fiziki 129, 86 (2006).
R. Sliter, M. Gish, and A. Vilesov, J. Phys. Chem. A 115, 9682 (2011).
D. J. Morre, et al., J. Inorg. Biochem. 102, 260 (2008).
S. V. Gudkov, V. I. Bruskov, M. E. Astashev, et al., J. Phys. Chem. B 115, 7693 (2011).
C. Huang, et al., Proc. Natl. Acad. Sci. USA 106, 15214 (2009).
W. C. Rontgen, Ann. d. Phys. u. Chem. N.F., 45, 91 (1891).
O. Ya. Samoilov, Structure of Water Solutions of Electrolytes and Ion Hydration (Izd. AN SSSR, Moscow, 1957) [in Russian].
L. Pauling, in Hydrogen Bonding, Ed. by D. Hadzi and H. W. Thompson (Pergamon Press, London, 1959), pp. 1–6.
M. Leetmaa, M. Ljungberg, H. Ogasawara, et al., J. Chem. Phys. 125, 244510 (2006).
P. Wernett, et al., Science 304, 995 (2004).
W. Chen, X. Wu, and R. Car, Phys. Rev. Lett. 105, 017802 (2010).
A. Nilsson and L. G. M. Pettersson, Chem. Phys. 389, 1 (2011).
G. N. I. Clark, G. L. Hura, J. Teixeira, et al., Proc. Natl. Acad. Sci. USA 107(32), 14003 (2010).
N. A. Bulienkov, Biofizika 36, 181 (1991).
V. I. Lobyshev, A. B. Solovei, and N. A. Bulienkov, Biophysics (Moscow) 48, 932 (2003).
A. B. Solovei, Candidate’s Dissertation (Moscow, 2006).
S. Ozeki and I. Otsuka, J. Phys. Chem. B 110, 20067 (2006).
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.D. Zakharov, 2013, published in Biofizika, 2013, Vol. 58, No. 5, pp. 904–909.
Rights and permissions
About this article
Cite this article
Zakharov, S.D. Ortho/para spin-isomers of H2O molecules as a factor responsible for formation of two structural motifs in water. BIOPHYSICS 58, 718–722 (2013). https://doi.org/10.1134/S0006350913050205
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350913050205