Abstract
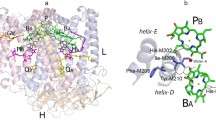
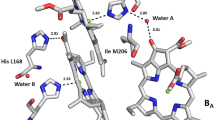
In the bacterial photosynthetic reaction center (RC), asymmetric protein environment of the bacteriochlorophyll (BChl) dimer largely determines the photophysical and photochemical properties of the primary electron donor. Previously, we noticed significant differences in properties of Rhodobacter sphaeroides RCs with identical mutations in symmetry-related positions — I(M206)H and I(L177)H. The substitution I(L177)H resulted in covalent binding of BChl PA with the L-subunit, as well as in 6-coordination of BChl BB, whereas in RC I(M206)H no such changes of pigment-protein interactions were found. In addition, the yield of RC I(M206)H after its isolation from membranes was significantly lower than the yield of RC I(L177)H. This study shows that replacement of amino acid residues in the M203–M206 positions near BChls PB and BA by symmetry-related residues from the L-subunit near BChls PA and BB leads to further decrease in RC amount in the membranes associated obviously with poor assembly of the complex. Introduction of a new hydrogen bond between BChl PB and its protein environment by means of the F(M197)H mutation stabilized the mutant RC but did not affect its low yield. We suggest that the mutation I(M206)H and substitution of amino acid residues in M203–M205 positions could disturb glycolipid binding on the RC surface near BChl BA that is important for stable assembly of the complex in the membrane.
Similar content being viewed by others
Abbreviations
- BA and BB :
-
monomer bacteriochlorophyll
- BChl:
-
bacteriochlorophyll
- LDAO:
-
lauryldimethylamine N-oxide
- P:
-
special pair of bacteriochlorophylls
- PA and PB :
-
bacteriochlorophylls of the special pair
- RC:
-
reaction center
- WT:
-
wild type
References
Allen, J. P., Feher, G., Yeates, T. O., Komiya, H., and Rees, D. S. (1987) Structure of reaction center from Rhodobacter sphaeroides R-26: the cofactors, PNAS, 84, 5730–5734.
Deisenhofer, J., and Michel, H. (1989) Nobel lecture. The photosynthetic reaction center from the purple bacterium Rhodopseudomonas viridis, EMBO, 8, 2149–2170.
Ermler, U., Fritzsch, G., Buchanan, S. K., and Michel, H. (1994) Structure of the photosynthetic reaction center from Rhodobacter sphaeroides at 2.65 Å resolution: cofactors and protein-cofactor interactions, Structure, 2, 925–936.
Camara-Artigas, A., Magee, C., Goetsch, A., and Allen, J. P. (2002) The structure of the heterodimer reaction center from Rhodobacter sphaeroides at 2.55 Å resolution, Photosynth. Res., 74, 87–93.
Vasilieva, L. G., Fufina, T. Y., Gabdulkhakov, A. G., Leonova, M. M., Khatypov, R. A., and Shuvalov, V. A. (2012) The site-directed mutation I(L177)H in Rhodobacter sphaeroides reaction center affects coordination of PA and BB bacteriochlorophylls, Biochim. Biophys. Acta, 1817, 1407–1417.
Potter, J. A., Fyfe, P. K., Frolov, D., Wakeham, M. C., van Grondelle, R., Robert, B., and Jones, M. R. (2005) Strong effects of an individual water molecule on the rate of primary charge separation in the Rhodobacter sphaeroides reaction center, J. Biol. Chem., 280, 27155–27164.
Yakovlev, A. G., Jones, M. R., Potter, J. A., Fyfe, P. K., Vasilieva, L. G., Shkuropatov, A. Y., and Shuvalov, V. A. (2005) Primary charge separation between P* and BA: electron-transfer pathways in native and mutant GM203L bacterial reaction centers, Chem. Phys., 319, 297–307.
Jones, M. R. (2007) Lipids in photosynthetic reaction centers: structural roles and functional holes, Progr. Lipid Res., 46, 56–87.
Leonova, M. M., Fufina, T. Y., Shuvalov, V. A., and Vasilieva, L. G. (2014) Study of pigment-protein interactions in the photosynthetic reaction center of the purple bacteria, in Modern Problems in Photosynthesis (Allakhverdiev, S. I., Rubin, A. B., and Shuvalov, V. A., eds.) [in Russian], Institute of Computer Studies, Moscow-Izhevsk, pp. 157–196.
Robles, S. J., Breton, J., and Youvan, D. C. (1990) Partial symmetrization of the photosynthetic reaction center, Science, 248, 1402–1405.
Taguchi, A. K. W., Stocker, J. W., Alden, R. G., Causgrove, T. P., Peloquin, J. M., Boxer, S. J., and Woodbury, N. W. (1992) Biochemical characterization and electron-transfer reactions of syml, a Rhodobacter capsulatus reaction center symmetry mutant which affects the initial electron donor, Biochemistry, 31, 10345–10355.
Hughes, A. V., Rees, P., Heathcote, P., and Jones, M. R. (2006) Kinetic analysis of the thermal stability of the photosynthetic reaction center from Rhodobacter sphaeroides, Biophys. J., 90, 4155–4166.
Jones, M. R. (2009) in Advances in Photosynthesis and Respiration, Vol. 28 (Hunter, C. N., Daldal, F., Thurnauer, M. C., and Beatty, J. T., eds.) Springer, pp. 295–321.
Holden-Dye, K., Crouch, L. I., Williams, C. M., Bone, R. A., Cheng, J., Bohles, F., Heathcote, P., and Jones, M. R. (2011) Opposing structural changes in two symmetrical polypeptides bring about opposing changes to the thermal stability of a complex integral membrane protein, Arch. Biochem. Biophys., 505, 160–170.
Bolgarina, T. I., Khatypov, R. A., Vasilieva, L. G., Shkuropatov, A. Y., and Shuvalov, V. A. (2004) Substitution of the Ile M206 by His in reaction center of Rhodobacter sphaeroides affects structure of the special pair bacteriochlorophyll, Doklady Biol. Sci., 394, 265–268.
Khatypov, R. A., Vasilieva, L. G., Fufina, T. U., Bolgarina, T. I., and Shuvalov, V. A. (2005) Biochemistry (Moscow), 70, 1256–1261.
Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A., Shkuropatov, A. Y., and Shuvalov, V. A. (2007) Substitution of isoleucine L177 by histidine in Rhodobacter sphaeroides reaction center results in the covalent binding of PA bacteriochlorophyll to the L subunit, FEBS Lett., 581, 5769–5773.
Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A., and Shuvalov, V. A. (2011) Properties of the Rhodobacter sphaeroides photosynthetic reaction center with double amino acid substitution I(L177)H + H(M182)L, Biochemistry (Moscow), 76, 450–454.
Van der Rest, M., and Gingras, G. (1974) The pigment complement of the photosynthetic reaction center isolated from Rhodospirillum rubrum, J. Biol. Chem., 249, 6446–6453.
Nolding, B. (2004) Methods in Modern Biophysics, Springer-Verlag, Berlin-Heidelberg.
Taguchi, A. K., Eastman, J. E., Gallo, D. M., Sheagley, E., Xiao, W., and Woodbury, N. W. (1996) Asymmetry requirements in the photosynthetic reaction center of Rhodobacter capsulatus, Biochemistry, 35, 3175–3186.
El-Kabbani, O., Chang, C. H., Tiede, D., Norris, J., and Schiffer, M. (1991) Comparison of reaction centers from Rhodobacter sphaeroides and Rhodopseudomonas viridis: overall architecture and protein-pigment interactions, Biochemistry, 30, 5361–5369.
Wakeham, M. C., Jones, M. R., Sessions, R. B., and Fyfe, P. K. (2001) Is there a conserved interaction between cardiolipin and the type II bacterial reaction center? Biophys. J., 80, 1395–1405.
Wakeham, M. C., Frolov, D., Fyfe, P. K., van Grondelle, R., and Jones, M. R. (2003) Acquisition of photosynthetic capacity by a reaction center that lacks the Q(A) ubiquinone; possible insights into the evolution of reaction centers, Biochim. Biophys. Acta, 1607, 53–63.
Sandberg, L., and Edholm, O. (2001) Calculated solvation free energies of amino acids in a dipolar approximation, J. Phys. Chem., 105, 273–281.
Fufina, T. Y., Vasilieva, L. G., Khatypov, R. A., and Shuvalov, V. A. (2013) Spectral properties of the Rhodobacter sphaeroides mutant photoreaction center with double amino acid substitution I(L177)H + H(L173)L, in Materials of the 15th Int. Conf. “Photosynthesis: Research for Food, Fuel, and Future, Adv. Top. Sci. Technol. China, pp. 46–49.
Vasilieva, L. G., Fufina, T. Y., Gabdulkhakov, A. G., Khatypov, R. A., and Shuvalov, V. A. (2014) Relocation BChl axial ligands in Rhodobacter sphaeroides mutant reaction centers, in Abstr. Int. Meet. “Photosynthesis Research for Sustainability”, Pushchino, Russia, p. 52.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2015, Vol. 80, No. 6, pp. 767–774.
Rights and permissions
About this article
Cite this article
Vasilieva, L.G., Fufina, T.Y., Gabdulkhakov, A.G. et al. Different effects of identical symmetry-related mutations near the bacteriochlorophyll dimer in the photosynthetic reaction center of Rhodobacter sphaeroides . Biochemistry Moscow 80, 647–653 (2015). https://doi.org/10.1134/S0006297915060012
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297915060012