Abstract
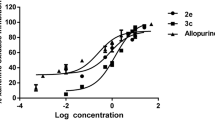
Inhibition of xanthine oxidase-catalyzed conversion of xanthine to uric acid by various pyrazolopyrimidine-based inhibitors (allopurinol derivatives) was evaluated and compared with the standard inhibitor allopurinol. Three compounds out of the seven compounds used in the study were found to be reasonably good inhibitors of xanthine oxidase (XO). 4-Amino-6-mercaptopyrazolo-3,4-d-pyrimidine was found to be the most potent inhibitor of XO (IC50=0.600±0.009 µM). 4-Mercapto-1H-pyrazolo-3,4-d-pyrimidine (IC50=1.326±0.013 µM) and 4-amino-6-hydroxypyrazolo-3,4-d-pyrimidine (IC50=1.564±0.065 µM) also showed inhibitory activity comparable to that of allopurinol (IC50 = 0.776 ± 0.012 µM). All three compounds showed competitive type of inhibition with comparable K i values. Induction of the electron transfer reaction catalyzed by XO in the presence of these compounds monitored as reduction of 2,6-dichlorophe nolindophenol (DCPIP) revealed that electron transfer by 4-amino-6-mercaptopyrazolo-3,4-d-pyrimidine is comparable to that obtained by allopurinol or xanthine. However, 4-mercapto-1H-pyrazolo-3,4-d-pyrimidine and 4-amino-6-hydroxypyrazolo-3,4-d-pyrimidine did not show DCPIP reduction. On the other hand, enzymatic reduction of cytochrome c in the presence of the three compounds was found to be insignificant and much less in comparison to allopurinol and xanthine. Therefore, both 4-amino-6-hydroxypyrazolo-3,4-d-pyrimidine and 4-mercapto-1H-pyrazolo-3,4-d-pyrimidine displayed the inhibitory property and also did not produce XO-mediated reactive oxygen species (ROS). Since 4-mercapto-1H-pyrazolo-3,4-d-pyrimidine was found to have some toxicity, the effect of 4-amino-6-hydroxypyrazolo-3,4-d-pyrimidine on the enzymatic formation of uric acid and ROS was investigated and it was found that this compound inhibited enzymatic generation of both uric acid and ROS. It can be noted that the standard inhibitor, allopurinol, inhibits uric acid formation but produces ROS.
Similar content being viewed by others
Abbreviations
- XO:
-
xanthine oxidase
- DCPIP:
-
2,6-dichlorophenolindophenol
- ROS:
-
reactive oxygen species
References
Hille, R., and Nishino, T. (1995) FASEB J., 9, 995–1003.
Krenitsky, T. A., Spector, T., and Hall, W. W. (1986) Arch. Biochem. Biophys., 247, 108–119.
Rodnan, G. P. (1982) Bull. Rheum. Dis., 32, 43–53.
McCord, J. M. (1985) N. Engl. J. Med., 312, 159–163.
Nakamura, M. (1991) J. Biochem. (Tokyo), 110, 450–456.
Massey, V., Komai, H., Palmer, G., and Elion, G. B. (1970) J. Biol. Chem., 245, 2837–2844.
Spector, T., and Johns, D. G. (1970) J. Biol. Chem., 245, 5079–5085.
Oettl, K., and Reibnegger, G. (1999) Biochim. Biophys. Acta, 1430, 387–395.
Okamoto, K., Eger, B. T., Nishino, T., Kondo, S., and Pai, E. F. (2003) J. Biol. Chem., 278, 1848–1855.
Ishibuchi, S., Morimoto, H., Oe, T., Ikebe, T., Inoue, H., Fukunari, A., Kamezawa, M., Yamada, I., and Naka, Y. (2001) Bioorg. Med. Chem. Lett., 11, 879–882.
Okamoto, K., Matsumoto, K., Hille, R., Eger, B. T., Pai, E. F., and Nishino, T. (2004) Proc. Natl. Acad. Sci. USA, 101, 7931–7936.
Lin, C. M., Chen, C. S., Chen, C. T., Liang, Y. C., and Lin, J. K. (2002) Biochem. Biophys. Res. Commun., 294, 167–172.
Van Hoom, D. E., Nijveldt, R. J., van Leeuwen, P. A., Hofman, Z., M’Rabet, L., De Bont, D. B., and van Norren, K. (2002) Eur. J. Pharmacol., 451, 111–118.
Tamta, H., Thilagavathi, R., Chakraborti, A. K., and Mukhopadhyay, A. K. (2005) J. Enzyme Inhibition Med. Chem., 20, 317–324.
Escribano, J., Gracia-Canovas, F., and Gracia-Carmona, F. (1988) Biochem. J., 254, 829–833.
Godner, B. L. J., Doel, J. J., Goult, T. A., Eisenthal, R., and Harrison, R. (2001) Biochem. J., 358, 325–333.
Hodges, G. R., Young, M. J., Paul, T., and Ingold, K. U. (2000) Free Rad. Biol. Med., 29, 434–441.
Liochev, S. I., and Fridovich, I. (2002) J. Biol. Chem., 277, 34674–34678.
Author information
Authors and Affiliations
Corresponding author
Additional information
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM05-106, October 2, 2005.
Rights and permissions
About this article
Cite this article
Tamta, H., Kalra, S. & Mukhopadhyay, A.K. Biochemical characterization of some pyrazolopyrimidine-based inhibitors of xanthine oxidase. Biochemistry (Moscow) 71 (Suppl 1), S49–S54 (2006). https://doi.org/10.1134/S0006297906130086
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1134/S0006297906130086