Abstract
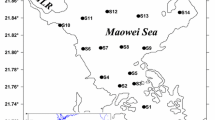
Studies of macrophytes in the coastal zone of the Artic Seas, including the White Sea, have shown the essential role of these algae in the activity of the coastal half-latitude ecosystems. In summer, during the macrophyte reproduction period, a great number of reproduction products are released into the water. For a short time, this considerably affects the ratio of the nanoplankton in the populations that inhibit the vast and shallow coastal areas. At different coastal sites in Chernorechenskaya Inlet, Kadalaksha Bay, during the period of intensive reproduction of Ascophyllum nodosum and Fucus vesiculosus, 42 plankton samples were collected in 2005. During this period the concentration of antherozoids in the water reached 55000 cells/ml (216 mg C/m3). The number of eggs was within the range of 0.05–0.7 cells/ml. The proportion of antherozoids in the total biomass of nanoplankton varied at different coastal sites from 0.37 to 99%, with a mean of 46% for the reproduction period of A. nodosum, and only 7% for the reproduction period of F. vesiculosus. As was shown by counts of F. vesiculosus female gametes in sedimentation traps, 1 m2 of the macrophyte bed (assuming 100% coverage) produces 18000–108000 eggs per day (0.33–2 mg C). The calculated flux of the reproductive material from the brown algae beds to the coastal water shows good agreement with the sample counts.
Similar content being viewed by others
References
E. I. Blinova, Macrophytic Algae and Grasses of Seas of the European Part of Russia (Flora, Distribution, Biology, Reseves, and Mariculture) (Izd. VNIRO, Moscow, 2007) [in Russian].
I. V. Burkovskii, Structural-Functional Organization and Stability of Marine Benthic Communities (as Exemplified by the Sandy Littoral of the White Sea) (Mosk. Gos. Univ., Moscow, 1992) [in Russian].
K. L. Vinogradova, “Division Rhodophyta (Red Algae),” in Life of Plants, Vol. 3: Algae. Lichens (Prosveshchenie, Moscow, 1977), pp. 192–250 [in Russian].
V. B. Vozzhinskaya, “Fucoids of the White Sea, Their Distribution, Biologyof Development, and Production,” in Basics of the Biological Productivity of the Ocean and Its Use (Nauka, Moscow, 1971), pp. 172–182 [in Russian].
V. B. Vozzhinskaya, Benthic Macrophytes of the White Sea (Nauka, Moscow, 1986) [in Russian].
V. B. Vozzhinskaya and A. N. Kamnev, Ecological-Biological Bases of Cultivation and Use of Marine Algae (Nauka, Moscow, 1994) [in Russian].
V. B. Vozzhinskaya, A. S. Tsapko, E. I. Blinova, et al., Commercially Important Algae of the USSR: A Handbook (Pishchevaya promyshlennost’, Moscow, 1971) [in Russian].
A Course in Lower Plants), Ed. by M. V. Gorlenko (Vysshaya shkola, Moscow, 1981) [in Russian].
A. A. Kalugina-Gutnik, Phytobenthos of the White Sea (Naukova dumka, Kiev, 1975) [in Russian].
A. N. Kamnev, Structure and Functions of Brown Algae (Mosk. Gos. Univ., Moscow, 1989) [in Russian].
V. V. Kuznetsov, The White Sea and the Biological Characteristics of Its Flora and Fauna (Akad. Nauk SSSR, Moscow, 1960) [in Russian].
L. L. Kuznetsov and E. V. Shoshina, Phytocenoses of the Barents Sea (Physiological and Structural Characteristics) (Izd. KNTs RAN, Apatity, 2003) [in Russian].
Yu. A. Mazei, Organization of the Microbenthos Community in the Zone of Mixing of River and Marine Waters, Candidate’s Dissertation in Biology (Mosk. Gos. Univ., Moscow, 2002) [in Russian].
O. A. Pronina, “Raw Resources and Harvest of Algae in the White Sea,” Rybnoe Khozyaistvo, No. 4, 44–47 (2002).
Z. P. Tikhovskaya, “Primary Production of Fucoids in Bays of Eastern Murman,” Tr. Murmansk. Biol. Stantsii 1, 164–188 (1948).
A. A. Udalov, V. O. Mokievskii, and E. S. Chertoprud, “Influence of the Salinity Gradient on the Distribution of Meiobenthos in the Chernaya River Estuary (White Sea),” Okeanologiya 45(5), 719–727 (2005) [Oceanology 45 (5), 680–688 (2005)].
K. M. Khailov, A. V. Prazukin, S. A. Kovardakov, and V. E. Rygalov, Functional Morphology of Marine Multicellular Algae (Naukova dumka, Kiev, 1992) [in Russian].
P. Aberg and H. Pavia, “Temporal and Multiple Scale Spatial Variation in Juvenile and Adult Abundance of the Brown Alga Ascophyllum nodosum,” Mar. Ecol. Progr. Ser. 158, 111–119 (1997).
S. Andersson, L. Kautsky, and A. Kalvas, “Circadian and Lunar Gamete Release in Fucus vesiculosus in the Atidal Baltic Sea,” Mar. Ecol. Progr. Ser. 110, 195–201 (1994).
P. O. Ang, “Natural Dynamics of a Fucus distichus (Phaeophyceae, Fucales) Population,” Mar. Ecol. Progr. Ser. 78, 71–85 (1991).
C. D. Armsler and R. B. Searles, “Vertical Distribution of Seaweed Spores in a Water Column Offshore of North Carolina,” J. Phycol. 16, 617–619 (1980).
S. Bäck, J. C. Collins, and G. Russel, Comparative Reproductive Biology of the Gulf of Finland and the Irish Sea Fucus vesiculosus L. Sarsia (London) 78 265–272 (1993).
L. C. Bacon and R. L. Vadas, “A Model for Gamete Release in Ascophyllum nodosum (Phaeophyta),” J. Phycol. 27(issue 2), 166–173 (1991).
R. Berger, T. Malm, and L. Kautsky, “Two Reproductive Strategies in Baltic Fucus vesiculosus (Phaeophyceae),” Eur. J. Phycol. 36(3), 265–273 (2001).
M.-L. Berndt, J. A. Callow, and S. H. Brawley, “Gamete Concentrations and Timing and Success of Fertilization in a Rocky Shore Seaweed,” Mar. Ecol. Progr. Ser. 226, 273–285 (2002).
E. Billard, E. A. Serrãp, G. A. Pearson, et al., “Analysis of Sexual Phenotype and Prezigotic Fertility in Natural Populations of Fucus spiralis, F. vesiculosus (Fucaceae, Phaeophyceae) and Their Putative Hybrids,” Eur. J. Phycol. 40, 397–407 (2005).
S. H. Brawley, “Fertilization in Natural Populations of the Dioecious Brown Alga Fucus ceranoides and the Importance of the Polyspermy Block,” Mar. Biol. 113, 145–157 (1992).
S. H. Brawley and L. E. Johnson, “Gametogenesis, Gametes and Zygotes: An Ecological Perspective on Sexual Reproduction in the Algae,” British Phycol. J. 27, 233–252 (1992).
D. A. Caron, “Technique for Enumeration of Heterotrophic Nanoplankton Using Epifluorescence Microscopy, and Comparison with Other Procedures,” App. Environ. Microbiol. 46, 491–498 (1983).
M. N. Clayton, “Propagules of Marine Macroalgae: Structure and Development,” Br. Phycol. J. 27, 219–232 (1992).
L. Deysher and T. A. Norton, “Dispersal and Colonization in Sargassum muticum (Yendo) Fensholt,” J. Exp. Mar. Biol. Ecol. 56, 179–195 (1982).
R. L. Fletcher and M. E. Callow, “The Settlement, Attachment and Establishment of Marine Algal Spores,” Br. Phycol. J. 27, 303–329 (1992).
H. Hillebrand, C.-D. Durselen, D. Kirschtel, et al., “Biovolume Calculation for Pelagic and Benthic Microalgae,” J. Phycol. 35, 403–424 (1999).
J. E. Hobbie, R. J. Daley, and S. Jasper, “Use of Nucleopore Filters for Counting Bacteria by Fluorescence Microscopy,” App. Environ. Microbiol. 33, 1225–1228 (1977).
A. C. Mathieson and Z. Guo, “Patterns of Fucoid Reproductive Biomass Allocation,” Br. Phycol. J. 27, 271–292 (1992).
S. Menden-Deuer and E. J. Lessard, “Carbon to Volume Relationship for Dinoflagellates, Diatoms, and Other Protist Plankton,” Limnol. Oceanogr. 45, 569–579 (2000).
J. C. Nejstgaard, L. J. Naustvoll, and A. F. Sazhin, “Correcting for Underestimation of Microzooplankton Grazing in Bottle Incubation Experiments with Mesozooplankton,” Mar. Ecol. Progr. Ser. 221, 59–75 (2001).
T. A. Norton, “Dispersal by Macroalgae,” Br. Phycol. J. 27, 293–301 (1992).
G. A. Pearson and S. H. Brawley, “Reproductive Ecology of Fucus distichus (Phaeophyceae): An Intertidal Alga with Successful External Fertilization,” Mar. Ecol. Progr. Ser. 143, 211–223 (1996).
G. A. Pearson and S. H. Brawley, “A Model for Signal Transduction during Gamete Release in the Fucoid Alga Pelvetia compressa,” Plant. Physiol. 118, 305–313 (1998).
G. A. Pearson, E. A. Serrão, and S. H. Brawley, “Control of Gamete Release in Fucoid Algae: Sensing Hydrodynamic Conditions via Carbon Acquisition,” Ecology 79(5), 1725–1739 (1998).
B. Robertson, Reproductive Ecology and Canopy Structure of Fucus spiralis Botanica Marina (London) XXX 475–482 (1987).
B. Santelices, “Recent Advances in Fertilization Ecology of Macroalgae,” J. Phycol. 38(issue 1), 4–10 (2002).
D. R. Schiel, “Algal Interactions on Subtidal Reefs in Northern New Zealand: A Review,” NZ J. Mar. Freshwat. Res. 22, 481–489 (1988).
E. A. Serrão, G. Pearson, L. Kautsky, and S. H. Brawley, “Successful External Fertilization in Turbulent Environments,” Proc. Natl. Acad. Sci. USA 93, 5286–5290 (1996).
S. Sutõ, “Studies on Shedding, Swimming and Fixing of the Spores of Seaweeds,” Bull. Japan Soc. Sci. Fish, No. 16, 1–9 (1950).
F. W. Zechman and A. C. Mathieson, “The Distribution of Seaweed Propagules in Estuarine, Coastal and Offshore Waters of New Hampshire, U.S.A,” Botanica Marina (London) XXVIII, 283–294 (1985).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Maximova, A.F. Sazhin, 2010, published in Okeanologiya, 2010, Vol. 50, No. 2, pp. 218–229.
Rights and permissions
About this article
Cite this article
Maximova, O.V., Sazhin, A.F. The role of gametes of the macroalgae Ascophyllum nodosum (L.) Le Jolis and Fucus vesiculosus L. (Fucales, Phaeophyceae) in summer nanoplankton of the White Sea coastal waters. Oceanology 50, 198–208 (2010). https://doi.org/10.1134/S0001437010020050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0001437010020050