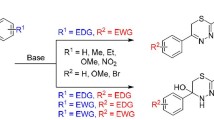
Abstract
The review summarizes published data on the reactions of cyclic and acyclic thioamides with derivatives of acetylenecarboxylic acids, which lead to the formation of both acyclic and heterocyclic systems, including thiazolidines and thiazines. Methods for determination of the structure of the condensation products and factors responsible for the reaction direction are discussed.
Similar content being viewed by others
References
Mushkalo, L.K. and Yangol, G.Ya., Ukr. Khim. Zh., 1955, vol. 21, p. 732.
Lown, J.W. and Ma, J.C.N., Can. J. Chem., 1967, vol. 45, p. 953.
Acheson, R.M. and Wallis, J.D., J. Chem. Soc., Perkin Trans. 1, 1981, p. 415.
Berseneva, V.S., Tkachev, A.V., Morzherin, Y.Y., Dehaen, W., Luyten, I., Toppet, S., and Bakulev, V.A., J. Chem. Soc., Perkin Trans. 1, 1998, p. 2133.
Kosterina, M.F., Morzherin, Yu.Yu., Kramarenko, O.A., Berseneva, V.S., Matern, A.I., Tkachev, A.V., and Bakulev, V.A., Russ. J. Org. Chem., 2004, vol. 40, p. 866.
Bloxham, J. and Dell, C.P., J. Chem. Soc., Perkin Trans. 1, 1994, p. 989.
Coen, S., Ragonnet, B., Vieillescazes, C., and Roggero, J.-P., Heterocycles, 1985, vol. 23, p. 1225.
Hendrickson, J.B., Rees, R., and Templeton, J.F., J. Am. Chem. Soc., 1964, vol. 86, p. 107.
Berseneva, V.S., Morzherin, Y.Y., Dehaen, W., Luyten, I., and Bakulev, V.A., Tetrahedron, 2001, vol. 57, p. 2179.
Beresneva, V.S., Biryucheva, N.Yu., and Bakulev, V.A., Khim. Geterotsikl. Soedin., 1993, p. 1688.
Pretsch, E., Clerc, Th., Seibl, J., and Simon, W., Tabellen zur Strukturaufklarung organischer Verbindunge, Berlin: Springer. 1976.
Hall, C.M. and Wemple, J., J. Org. Chem., 1977, vol. 42, p. 2118.
Short, F.W., Littleton, B.C., and Johnson, J.L., Chem. Ind., 1971, p. 705.
Hiroshi, N., Chem. Pharm. Bull., 1973, vol. 21, p. 270.
Van der Vliet, P.N.W., Hamersma, J.A.M., and Speckamp, W.N., Tetrehedron, 1985, vol. 41, p. 2007.
Kataev, E.G., Konovalova, L.K., and Yarkova, E.G., Zh. Org. Khim., 1968, vol. 5, p. 621.
Gianolla, L.I., Palazzo, S., Agozzino, P., Lamartina, L., and Ceraulo, L., J. Chem. Soc., Perkin Trans. 1, 1978, p. 1428.
Kihiola, Y. and Teraola, A., Chem. Pharm. Bull., 1968, vol. 16, p. 1351.
Lown, J.L. and Ma, J.C.N., Can. J. Chem., 1967, vol. 45, p. 939.
Winterfeld, E. and Nelke, J.M., Chem. Ber., 1967, vol. 100, p. 3671.
Vögeli, U., von Philipsborn, W., Nagarajan, K., and Nair, M.D., Helv. Chim. Acta, 1978, vol. 61, p. 607.
Moriyama, H., Yakugaku Zasshi, 1963, vol. 83, p. 169; Chem. Abstr., 1963, vol. 59, p. 3925.
Sasaki, H., Sakata, H., and Iwanami, Y., Nippon Kagaku Zasshi, 1964, vol. 85, p. 704; Chem. Abstr., 1965, vol. 62, p. 14678.
Akerblom, E., Chem. Scripta, 1974, vol. 6, p. 35.
Dallas, G., Lown, J.W., and Ma, J.C.N., J. Chem. Soc. C, 1968, p. 2510.
Cain, E.N. and Warrener, R.N., Aust. J. Chem., 1970, vol. 23, p. 51.
Tanaka, H. and Yohoyama, A., Chem. Pharm. Bull., 1962, vol. 10, p. 19.
Janietz, D., Goldmann, B., and Rudorf, W.-D., J. Prakt. Chem., 1988, vol. 330, p. 607.
Hurst, D.T., Atcha, S., and Marshall, K.L., Aust. J. Chem., 1991, vol. 44, p. 129.
Adman, E., Jensen, L.H., and Warrener, R.N., Acta Crystallogr., Sect. B, 1975, vol. 31, p. 1915.
Cameron, A.F. and Hair, N.J., J. Chem. Soc. B, 1971, p. 1733.
Cameron, A.F., Hair, N.J., Elmore, N.F., and Taylor, P.J., J. Chem. Soc., Chem. Commun., 1970, p. 890.
Behringer, H., Bender, D., Falkenberg, J., and Wiedenmann, R., Chem. Ber., 1968, vol. 101, p. 1428.
Günther, H., NMR Spectroscopy: An Introduction, Chichester: Wiley, 1980.
Danilkina, N.A., Mikhailov, L.E., Ganina, M.B., and Ivin, B.A., Russ. J. Gen. Chem., 2004, vol. 74, p. 148.
Danilkina, N.A., Vershilov, S.V., Ganina, M.B., Mikhailov, L.E., and Ivin, B.A., Russ. J. Gen. Chem., 2004, vol. 74, p. 472.
Hiroshi, N., Chem. Pharm. Bull., 1973, vol. 21, p. 279.
Kauss, V.Ya., Liepin’sh, E.E., Kalvin’sh, I.Ya., and Lukevits, E., Khim. Geterotsikl. Soedin., 1990, p. 120.
El-Fattah, B. Abo., Al-Ashmawi, M.I., El-Feky, S., and Roeder, E., Chung-hua Yoa Hsueh Tsa Chih., 1991, vol. 43, p. 129; Chem. Abstr., 1991, vol. 115, no. 92 191y.
Vas’kevich, R.I., Zborovskii, Yu.L., Staninets, V.I., and Chernega, A.N., Russ. J. Org. Chem., 2004, vol. 40, p. 1047.
Kurasawa, Y., Katoh, R., Takada, A., Kim, H.S., and Okamoto, Y., J. Heterocycl. Chem., 1992, vol. 29, p. 1001.
Kauss, V.Ya., Mishnev, A.F., and Kalvin’sh, I.Ya., Khim. Geterotsikl. Soedin., 1992, p. 1674.
Adjon, A., Tea, C.G., and Seikou, T.A., J. Soc. Ouest-Afr. Chim., 1998, vol. 3, p. 11; Chem. Abstr., 1999, vol. 131, no. 296 655f.
Garraway, J.L., J. Chem. Soc., 1962, p. 4077.
Warrener, R.N. and Cain, E.N., Chem. Ind., 1964, p. 1989.
Osborne, N.F., J. Chem. Soc., Perkin Trans. 1, 1980, p. 146.
Grinblat, E.I. and Postovskii, I.Ya., Zh. Org. Khim., 1961, vol. 31, p. 394.
McKillop, A., Bellinger, G.C.A., Preston, P.N., Davidson, A., and King, T.J., Tetrahedron Lett., 1978, vol. 19, p. 2621.
Nagarajan, K., Nair, M.D., and Desai, J.A., Tetrahedron Lett., 1979, vol. 20, p. 53.
Bakulev, V.A., Berseneva, V.S., Belskaia, N.P., Morzherin, Y.Y., Tkachev, A.V., Dehaen, W., Luyten, I., and Toppet, S., Org. Biomol. Chem., 2003, p. 134.
Ishida, N. and Imafuku, K., Phosporus, Sulfur Silicon Relat. Elem., 1996, vol. 108, p. 239.
Claiton, J.P., O’Hanlon, P.J., and King, T.J., J. Chem. Soc. Perkin Trans. 1, 1980, p. 1352.
Nair, M.D., Nagarajan, K., and Desai, J.A., Indian J. Chem., Sect. B, 1979, vol. 18, p. 479.
Al-Jallo, H.N. and Muniem, M.A., J. Heterocycl. Chem., 1978, vol. 15, p. 849.
Giammona, G., Neri, M., Carlis, B., Palazzo, A., and La Rosa, C., J. Heterocycl. Chem., 1991, vol. 28, p. 325.
Heravi, M.M., Beheshfiha, Y.Sh., Oskooie, H.A., and Nami, N., Indian J. Heterocycl. Chem., 1999, vol. 8, p. 245; Chem. Abstr., 1999, vol. 131, no. 102 244y.
Upadhyaya, V.P. and Srinivasan, V.R., Indian J. Chem., Sect. B, 1978, vol. 16, p. 737.
Britsun, V.N., Esipenko, A.N., Chernega, A.N., and Lozinskii, M.O., Russ. J. Org. Chem., 2005, vol. 41, p. 108.
Gogoi, P.C. and Kataky, J.C.S., Heterocycles, 1991, vol. 32, p. 231.
Ozbey, S. and Tozkoparan, B., Acta Crystallogr., Sect. F, 2002, vol. 58, p. 1193.
Heravi, M.M., Montazeri, N., Rahimizadeh, M., Bakavoli, M., and Ghassemzadeh, M., Monatsh. Chem., 2001, vol. 132, p. 1225
Heindel, N.D. and Reid, J.R., J. Heterocycl. Chem., 1980, vol. 17, p. 1087.
Gogoi, P.C. and Kataky, J.C.S., Heterocycles, 1990, vol. 31, p. 2147.
Heravi, M.M., Rahimizadeh, M., Dawoodnia, A., Seyf, M., and Ghassemzadeh, M., Phosphorus, Sulfur Silicon Relat. Elem., 2000, pp. 167, 211.
Feky, El., Said, A.H., Samii, A., Zakaria, K.M., and Jaeda, M., Alexandria J. Pharm. Sci., 1990, vol. 4, p. 117; Chem. Abstr., 1991, vol. 114, no. 143 371e.
Simó, M., Csámpai, A., Harmat, V., Barabás, O., and Magyarfalvy, G., Tetrahedron, 2001, vol. 57, p. 7191.
Heindel, N.D. and Reid, J.R., J. Org. Chem., 1980, vol. 45, p. 2479.
Heravi, M.M., Beheshfiha, Y.Sh., Nami, N., and Ghassemzadeh, M., Phosphorus, Sulfur Silicon Relat. Elem., 2000, vol. 161, p. 71.
Giannola, L.I., Palazzo, S., Lamartina, L., di Sanseverino, L.R., and Sabatino, P., J. Chem. Soc., Perkin Trans. 1, 1986, p. 2095.
Sohár, P., Szöke-Molnar, Z., Stájer, G., and Bernáth, G., Magn. Reson. Chem., 1989, vol. 27, p. 959.
Stájer, G., Szabó, A.E., and Sohár, P., Heterocycles, 1999, vol. 51, p. 1849.
Yao Chung-huo, Chin Hsueh Tsa, Chien Lio Kang, Kuan Hu Ming, Chung-hua Yoa Hsueh Tsa Chih., 1990, vol. 42, p. 83; Chem. Abstr., 1991, vol. 114, no. 23 910h.
Sibor, J., Pfeiffer, W.D., Hetzheim, A., and Pazdera, P., Scr. Chem., 1997–1998, vols. 27–28, p. 33; Chem. Abstr., 2000, vol. 132, no. 23 022s.
Heravi, M.M., Beheshfiha, Y.Sh., Shoar, R.H., and Nami, N., Phosphorus, Sulfur Silicon Relat. Elem., 2000, vol. 165, p. 285.
Giannola, L.I., Giammona, D., Palazzo, S., and Lamartina, L., J. Chem. Soc., Perkin Trans. 1, 1984, p. 2707.
Heravi, M.M., Kazemi, A., Tajbakhsh, M., Nooshabadi, M.A., and Mojtahedi, M.M., Indian J. Heterocycl. Chem., 2000, vol. 10, p. 151; Chem. Abstr., 2001, vol. 134, no. 295 886c.
Heravi, M.M., Oskooie, H.A., Behesfiha, Y.Sh., Nami, N., and Ghovesishi, S., Indian J. Heterocycl. Chem., 1998, vol. 7, p. 303; Chem. Abstr., 1998, vol. 129, no. 230 703t.
Heravi, M.M., Montazeri, N., Raahimizadeh, M., Bakavoli, M., and Ghassemzadeh, M., Phosphorus, Sulfur Silicon Relat. Elem., 2001, vol. 170, p. 187.
Author information
Authors and Affiliations
Additional information
Original Russian Text © N.A. Danilkina, L.E. Mikhailov, B.A. Ivin, 2006, published in Zhurnal Organicheskoi Khimii, 2006, Vol. 42, No. 6, pp. 807–839.
Natal’ya Danilkina graduated from the Pharmaceutical Faculty, St. Petersburg Chemical and Pharmaceutical Academy, in 2003 and entered post-graduate courses at the Organic Chemistry Department, St. Petersburg Chemical and Pharmaceutical Academy. In 2004, she was a J. Soros’ post-graduate student. Fields of scientific interest: chemistry of heterocyclic compounds and application of NMR for studying the structure of heterocycles.
Leonid Mikhailov graduated from the Faculty of Chemistry, Leningrad State University, in 1989. In 1995–1998, he was a post-graduate student at the Organic Chemistry Department, St. Petersburg Chemical and Pharmaceutical Academy, where he sustained his Candidate’s Theses in 1998. L. Mikhailov works now at the Department of Dyes and Phototropic Compounds, St. Petersburg State Institute of Technology. Field of scientific interest: chemistry of biologically active heterocyclic compounds.
Boris Ivin is Doctor of Chemical Sciences, Professor, Honored Scientific Worker of the Russian Federation, Head of the Organic Chemistry Department, St. Petersburg Chemical and Pharmaceutical Academy. Fields of scientific interest: chemistry of heterocyclic compounds, medicinal chemistry, and relations between the structure and biological activity of organic compounds.
Rights and permissions
About this article
Cite this article
Danilkina, N.A., Mikhailov, L.E. & Ivin, B.A. Condensations of thioamides with acetylenecarboxylic acid derivatives. Russ J Org Chem 42, 783–814 (2006). https://doi.org/10.1134/S1070428006060017
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1070428006060017