Abstract
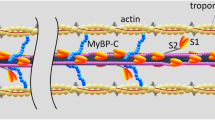
The force exerted by skeletal muscle is modulated by compliance of tissues to which it is connected. Force of the muscle sarcomere is modulated by compliance of the myofilaments. We tested the hypothesis that myofilament compliance influences Ca2+ regulation of muscle by constructing a computational model of the muscle half sarcomere that includes compliance of the filaments as a variable. The biomechanical model consists of three half-filaments of myosin and 13 thin filaments. Initial spacing of motor domains of myosin on thick filaments and myosin-binding sites on thin filaments was taken to be that measured experimentally in unstrained filaments. Monte-Carlo simulations were used to determine transitions around a three-state cycle for each cross-bridge and between two-states for each thin filament regulatory unit. This multifilament model exhibited less “tuning” of maximum force than an earlier two-filament model. Significantly, both the apparent Ca2+-sensitivity and cooperativity of activation of steady-state isometric force were modulated by myofilament compliance. Activation-dependence of the kinetics of tension development was also modulated by filament compliance. Tuning in the full myofilament lattice appears to be more significant at submaximal levels of thin filament activation.
Similar content being viewed by others
REFERENCES
Adams, G. R., V. J. Caiozzo, and K. M. Baldwin. Skeletal muscle unweighting: Spaceflight and ground-based models. J. Appl. Physiol. 95:2185–2201, 2003.
Bagshaw, C. R. Muscle Contraction. London: Chapman & Hall, 1993.
Brandt, P. W., M. S. Diamond, and F. H. Schachat. The thin filament of vertebrate skeletal muscle co-operatively activates as a unit. J. Mol. Biol. 180:379–384, 1984.
Brenner, B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: Implications for regulation of muscle contraction. Proc. Natl. Acad. Sci. U.S.A. 85:3265–3269, 1988.
Brenner, B., and E. Eisenberg. Rate of force generation in mus-cle: Correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. U.S.A. 83:3542–3546, 1986.
Bukatina, A. E., and F. Fuchs. Effect of phalloidin on the ATPase activity of striated muscle myofibrils. J. Muscle Res. Cell Motil. 15:29–36, 1994.
Bukatina, A. E., F. Fuchs, and P. W. Brandt. Thin filament ac-tivation by phalloidin in skinned cardiac muscle. J. Mol. Cell. Cardiol. 27:1311–1315, 1995.
Campbell, K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys. J. 72:254–262, 1997.
Chase, P. B., M. Macpherson, and T. L. Daniel. A spatially explicit, 3-D model of the muscle sarcomere. Biophys. J. 82:5a, 2002.
Chase, P. B., D. A. Martyn, and J. D. Hannon. Isometric force redevelopment of skinned muscle fibers from rabbit with and without Ca2+. Biophys. J. 67:1994–2001, 1994.
Daniel, T. L., A. C. Trimble, and P. B. Chase. Compliant re-alignment of binding sites in muscle: Transient behavior and mechanical tuning. Biophys. J. 74:1611–1621, 1998.
Daniel, T. L., and M. S. Tu. Animal movement, mechanical tuning and coupled systems. J. Exp. Biol. 202 Pt 23:3415–3421, 1999.
Farley, C. T., J. Glasheen, and T. A. McMahon. Running springs: Speed and animal size. J. Exp. Biol. 185:71–86, 1993.
Fatkin, D., and R. M. Graham. Molecular mechanisms of inherited cardiomyopathies. Physiol. Rev. 82:945–980, 2002.
Gafurov, B.,S. Fredricksen, A. Cai, B. Brenner, P.B. Chase, and J. M. Chalovich. The 14 mutant of troponin T enhances ATPase activity and alters the cooperative binding of S1-ADP to regulated actin. Biochemistry, In press.
Gittes, F., B. Mickey, J. Nettleton, and J. Howard. Flexural rigid-ity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120:923–934, 1993.
Gordon, A. M., E. Homsher, and M. Regnier. Regulation of contraction in striated muscle. Physiol. Rev. 80:853–924, 2000.
Hancock, W. O., L. L. Huntsman, and A. M. Gordon. Models of calcium activation account for differences between skeletal and cardiac force redevelopment kinetics. J. Muscle Res. Cell Motil. 18:671–681, 1997.
Higuchi, H., T. Yanagida, and Y. E. Goldman. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Bio-phys. J. 69:1000–1010, 1995.
Howard, J. Mechanics of Motor Proteins and the Cytoskeleton. Sunderland, MA: Sinaur Associates, 2001.
Huxley, H. E., A. Stewart, H. Sosa, and T. Irving. X-ray diffrac-tion measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys. J. 67:2411–2421, 1994.
Isambert, H., P. Venier, A. C. Maggs, A. Fattoum, R. Kassab, D. Pantaloni, and M.-F. Carlier. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J. Biol. Chem. 270:11437–11444, 1995.
Köhler, J., Y. Chen, B. Brenner, A. M. Gordon, T. Kraft, D. A. Martyn, M. Regnier, A. J. Rivera, C.-K. Wang, and P. B. Chase. Familial hypertrophic cardiomyopathy mutations in troponin I (K183Δ, G203S, K206Q) enhance filament sliding. Physiol. Genomics 14:117–128, 2003.
Lambeth, M. J., and M. J. Kushmerick. A computational model for glycogenolysis in skeletal muscle. Ann. Biomed. Eng. 30:808–827, 2002.
Linari, M., I. Dobbie, M. Reconditi, N. Koubassova, M. Irving, G. Piazzesi, and V. Lombardi. The stiffness of skele-tal muscle in isometric contraction and rigor: The fraction of myosin heads bound to actin. Biophys. J. 74:2459–2473, 1998.
Luo, Y., R. Cooke, and E. Pate. A model of stress relaxation in cross-bridge systems: Effect of a series elastic element. Am. J. Physiol. 265:C279–C288, 1993.
Luo, Y., R. Cooke, and E. Pate. Effect of series elasticity on delay in development of tension relative to stiffness during muscle activation. Am.J.Physiol.267:C1598–C1606, 1994.
Martyn, D. A., and P. B. Chase. Faster force transient kinetics at submaximal Ca2+ activation of skinned psoas fibers from rabbit. Biophys. J. 68:235–242, 1995.
Martyn, D. A., P. B. Chase, M. Regnier, and A. M. Gordon. A simple model with myofilament compliance predicts activation dependent cross-bridge kinetics in skinned skeletal fibers. Biophys. J. 83:3425–3434, 2002.
McMahon, T. A., and P. R. Greene. The influence of track compliance on running. J. Biomech. 12:893–904, 1979.
Mijailovich, S. M., J. J. Fredberg, and J. P. Butler. On the theory of muscle contraction: Filament extensibility and the development of isometric force and stiffness. Biophys. J. 71:1475–1484, 1996.
Millman, B. M. The filament lattice of striated muscle. Physiol. Rev. 78:359–391, 1998.
Molloy, J. E., J. E. Burns, J. Kendrick-Jones, R. T. Tregear, and D. C. S. White. Movement and force produced by a single myosin head. Nature 378:209–212, 1995.
Molloy, J.E.,J.E. Burns, J.C. Sparrow, R.T. Tregear, J. Kendrick-Jones, and D. C. S. White. Single-molecule mechan-ics of heavy meromyosin and S1 interacting with rabbit or Drosophila actins using optical tweezers. Biophys. J. 68:298S–303S, S-5S, 1995.
Regnier, M., D. A. Martyn, and P. B. Chase. Calmidazolium alters Ca2+ regulation of tension redevelopment rate in skinned skeletal muscle. Biophys. J. 71:2786–2794, 1996.
Regnier, M., D. A. Martyn, and P. B. Chase. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys. J. 74:2005–2015, 1998.
Regnier, M., A. J. Rivera, M. A. Bates, C.-K. Wang, P. B. Chase, and A. M. Gordon. Thin filament near-neighbor regulatory unit interactions affect rabbit skeletal muscle steady state force-Ca2+ relations. J. Physiol. 540:485–497, 2002.
Regnier, M., A. J. Rivera, P. B. Chase, L. B. Smillie, and M. M. Sorenson. Regulation of skeletal muscle tension redevelopment by troponin C constructs with different Ca2+ affinities. Biophys. J. 76:2664–2672, 1999.
Riley, D. A., J. L. Bain, J. L. Thompson, R. H. Fitts, J. J. Widrick, S. W. Trappe, T. A. Trappe, and D. L. Costill. Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J. Appl. Physiol. 88:567–572, 2000.
Salem, J.E.,G.M. Saidel, W.C. Stanley,and M.E. Cabrera. Mechanistic model of myocardial energy metabolism under nor-mal and ischemic conditions. Ann. Biomed. Eng. 30:202–216, 2002.
Sweeney, H. L., and J. T. Stull. Alteration of cross-bridge ki-netics by myosin light chain phosphorylation in rabbit skeletal muscle: Implications for regulation of actin-myosin interaction. Proc. Natl. Acad. Sci. U.S.A. 87:414–418, 1990.
Veigel, C., M. L. Bartoo, D. C. S. White, J. C. Sparrow, and J. E. Molloy. The stiffness of rabbit skeletal actomyosin cross-bridges determined with an optical tweezers transducer. Biophys. J. 75:1424–1438, 1998.
Wakabayashi, K., Y. Sugimoto, H. Tanaka, Y. Ueno, Y. Takezawa, and Y. Amemiya. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys. J. 67:2422–2435, 1994.
White, R. J., and M. Averner. Humans in space. Nature 409:1115–1118, 2001.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chase, P.B., Macpherson, J.M. & Daniel, T.L. A Spatially Explicit Nanomechanical Model of the Half-Sarcomere: Myofilament Compliance Affects Ca2+-Activation. Annals of Biomedical Engineering 32, 1559–1568 (2004). https://doi.org/10.1114/B:ABME.0000049039.89173.08
Issue Date:
DOI: https://doi.org/10.1114/B:ABME.0000049039.89173.08