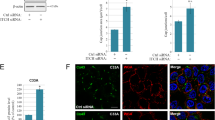
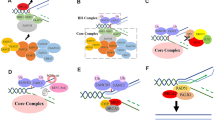
Abstract
The adenomatous polyposis coli (Apc) gene is mutated in familial adenomatous polyposis and in sporadic colorectal tumors. The Apc gene product (APC), basically a cytoplasmic protein, blocks cell cycle progression and plays crucial roles in development. The APC binds to β-catenin, axin and glycogen synthase kinase 3β to form a large protein complex, in which β-catenin is phosphorylated and broken down, resulting in negative regulation of the Wnt signaling pathway. Most of the mutated Apc genes in colorectal tumors lack β-catenin-binding regions and fail to inhibit Wnt signaling, leading to overproliferation of tumor cells. The APC, having some nuclear localizing signals in its molecule, can also be localized in the nucleus. The nuclear APC exports excess β-catenin to the cytoplasm. Through its C-terminus, APC binds to post-synaptic density discs large zonula occludens domain-containing proteins, such as discs large (DLG) and post-synaptic density (PSD)-95, and may play important roles in epithelial morphogenesis, brain development and neuronal functions. In addition, APC is involved in cell motility through its association with microtubules and APC-stimulated guanine nucleotide exchange factor. Colocalization of APC and DLG is dependent on microtubules. The Apc gene is highly expressed in the embryonic and postnatal developing brain. Recently, we found that APC is required for the activity of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by facilitating the clustering of PSD-95 and these receptors at the postsynapse. In addition, APC is present in astrocytes, although its role in astrocytes is, as yet, unknown.
Similar content being viewed by others
References
Baeg GH, Matsumine A, Kuroda T et al. (1995) The tumour suppressor gene product APC blocks cell cycle progression from G0/G1 to S phase. EMBO J 14, 5618–25.
Behrens J, Jerchow BA, Wurtele M et al. (1998) Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3 beta. Science 280, 596–9.
Bhat RV, Baraban JM, Johnson RC, Eipper BA, Mains RE (1994) High levels of expression of the tumor suppressor gene APC during development of the rat central nervous system. J Neurosci 14, 3059–71.
Bodmer WF, Baily CJ, Bodmer J et al. (1987) Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature 328, 614–16.
Brakeman JSF, Gu SH, Wang XB, Dolin G, Baraban JM (1999) Neuronal localization of the adenomatous polyposis coli tumor suppressor protein. Neuroscience 91, 661–72.
Cadigan KM, Nusse R (1997) Wnt signaling: A common theme in animal development. Genes Dev 11, 3286–305.
Fearnhead NS, Britton MP, Bodmer WF (2001) The ABC of APC. Hum Mol Genet 10, 721–33.
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61, 759–67.
Fodde R, Edelmann W, Yang K et al. (1994) A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proc Natl Acad Sci USA 91, 8969–73.
Fodde R, Kuipers J, Rosenberg C et al. (2001) Mutations in the APC tumor suppressor gene cause chromosomal instability. Nat Cell Biol 3, 433–8.
Groden J, Thliveris A, Samowitz W et al. (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66, 589–600.
Hamada F, Tomoyasu Y, Takatsu Y et al. (1999) Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science 283, 1739–42.
Hamilton SR, Liu B, Parsons RE et al. (1995) The molecular basis of Turcot’s syndrome. N Engl J Med 332, 839–47.
Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P (1998) Down regulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 8, 573–81.
He T-C, Sparks AB, Rago C et al. (1998) Identification of c-MYC as a target of the APC pathway. Science 281, 1509–12.
Henderson BR (2000) Nuclear-cytoplasmic shuttling of APC regulates beta-catenin suncellular localization and turnover. Nat Cell Biol 2, 653–60.
Herrera L, Kakati S, Gibas L, Pietrzak E, Sandberg A (1986) Brief clinical report. Gardner syndrome in a man with an interstitial deletion of 5q. Am J Genet 25, 473–6.
Hülsken J, Birchmeier W, Behrens J (1994) E-cadherin and APC compete for the interaction with β-catenin and cytoskeleton. J Cell Biol 127, 2061–9.
Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A (1998) Axin, a negative regulator of the Wnt signaling pathway, forms complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J 17, 1371–84.
Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A (2000) GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene 19, 537–45.
Ishidate T, Matsumine A, Toyoshima K, Akiyama T (2000) The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 19, 365–72.
Jimbo T, Kawasaki Y, Koyama R et al. (2002) Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol 4, 323–7.
Joslyn G, Carlson M, Thliveris A et al. (1991) Identification of deletion mutations and three new genes at the familial polyposis locus. Cell 66, 601–3.
Kakinuma N, Nishimura Y, Akiyama T, Senda T (2000) APC is colocalized with β-catenin and hDLG in Henle’s loop of the mouse kidney. Acta Histochem Cytochem 33, 457–63.
Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Näthke IS (2001) A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol 3, 429–32.
Kawasaki Y, Senda T, Ishidate T et al. (2000) Asef, a link between the tumor suppressor APC and G-protein signaling. Science 289, 1194–7.
Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87, 159–70.
Kinzler KW, Nilbert MC, Su LK et al. (1991) Identification of FAP locus genes from chromosome 5q21. Science 253, 661–5.
Kishida S, Yamamoto H, Ikeda S et al. (1998) Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem 273, 10 823–6.
Knudson Jr AG (1971) Mutations and cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci USA 68, 820–3.
Iizuka-Kogo A, Shimomura A, Senda T (2005) Colocalization of APC and DLG at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons. Histochem Cell Biol 123, 67–73.
Korinek V, Barker N, Morin PJ et al. (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC+ colon carcinoma. Science 275, 1784–7.
Leppert M, Dobbs M, Scambler P et al. (1987) The gene for familial polyposis coli maps to the long arm of chromosome 5. Science 238, 1411–13.
Matsumine A, Ogai A, Senda T et al. (1996) Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 272, 1020–3.
Miller JR, Moon RT (1996) Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev 10, 2527–39.
Mimori-Kiyosue Y, Shiina N, Tsukita S (2000) Adenomatous polyposis coli (APC) protein moves along microtubules and concentrates at their growing ends in epithelial cells. J Cell Biol 148, 505–17.
Miyashiro I, Senda T, Matsumine A et al. (1995) Subcellular localization of the APC protein: Immunoelectron microscopic study of the association of the APC protein with catenin. Oncogene 11, 89–96.
Morin PJ, Sparks AB, Korinek V et al. (1997) Activation of β-catenin-Tcf signaling in colon cancer by mutations in β- catenin or APC. Science 275, 1787–90.
Moser AR, Pitot HC, Dove WF (1990) A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–4.
Moser AR, Shoemaker AR, Connelly CS et al. (1995) Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev Dyn 203, 422–33.
Munemitsu S, Souza B, Müller O, Albert I, Rubinfeld B, Polakis P (1994) The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res 54, 3676–81.
Nakamura T, Hamada F, Ishidate T et al. (1998) Axin, an inhibitor of the Wnt signaling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cells 3, 395–403.
Näthke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ (1996) The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol 134, 165–79.
Neufeld KL, White RL (1997) Nuclear and cytoplasmic localizations of the adenomatous polyposis coli protein. Proc Natl Acad Sci USA 94, 3034–9.
Nishisho I, Nakamura Y, Miyoshi Y et al. (1991) Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253, 665–9.
Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M (1995) Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci USA 92, 4482–6.
Oshima M, Dinchuk JE, Kargman SL et al. (1996) Suppression of intestinal polyposis in Apeδ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87, 803–9.
Peifer M (1997) β-Catenin as oncogene. The smoking gun. Science 275, 1752–3.
Powell SM, Zilz N, Beazer-Barclay Y et al. (1992) APC mutations occur early during colorectal tumorigenesis. Nature 359, 235–7.
Rosin-Arbesfeld R, Townsley F, Bienz M (2000) The APC tumour suppressor has a nuclear export function. Nature 406, 1009–12.
Rubinfeld B, Souza B, Albert I et al. (1993) Association of the APC gene product with β-catenin. Science 262, 1731–4.
Satoh K, Yanai H, Senda T et al. (1997) DAP-1, a novel protein that interacts with the guanylate kinase-like domains of hDLG and PSD-95. Genes Cells 2, 415–24.
Senda T, Miyashiro I, Matsumine A et al. (1996) The tumor suppressor protein APC colocalizes with β-catenin in the colon epithelial cells. Biochem Biophys Res Commun 223, 329–34.
Senda T, Iino S, Matsushita K, Matsumine A, Kobayashi S, Akiyama T (1998) Localization of the adenomatous polyposis coli tumour suppressor protein in the mouse central nervous system. Neuroscience 83, 857–66.
Shibata H, Toyama K, Shioya H et al. (1997) Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278, 120–3.
Shimomura A, Kohu K, Akiyama T, Senda T (2005) Subcellular localization of the tumor suppressor APC in developing cultured neurons. Neurosci Lett 375, 81–6.
Smith KJ, Johnson KA, Bryan TM et al. (1993) The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA 90, 2846–50.
Smith KJ, Levy DB, Maupin P, Pollard TD, Vogelstein B, Kinzler KW (1994) Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res 54, 3672–5.
Smits R, van der Houven van Oordt W, Luz A et al. (1998) Apc1638N: A mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology 114, 275–83.
Smits R, Kielman MF, Breukel C (1999) Apc1638T: A mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 13, 1309–21.
Su LK, Kinzler KW, Vogelstein B et al. (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–70.
Su LK, Vogelstein B, Kinzler KW (1993) Association of the APC tumor suppressor protein with catenins. Science 262, 1734–7.
Su LK, Burrell M, Hill DE et al. (1995) APC binds to the novel protein EB1. Cancer Res 55, 2972–7.
Tago K, Nakamura T, Nishita M et al. (2000) Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev 14, 1741–9.
Temburni MK, Rosenberg MM, Pathak N, McConnell R, Jacob MH (2004) Neuronal nicotinic synapse assembly requires the adenomatous polyposis coli tumor suppressor protein. J Neurosci 24, 6776–84.
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–6.
Turcot J, Despres JP, St Pierre F (1959) Malignant tumors of the central nervous system associated with familial polyposis of the colon: Report of two cases. Dis Colon Rectum 2, 465–8.
Wang J, Jing Z, Zhang L et al. (2003) Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neuro sci 6, 1017–18.
Wong MH, Hermiston ML, Syder AJ, Gordon JI (1996) Forced expression of the tumor suppressor adenomatous polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc Natl Acad Sci USA 93, 9588–93.
Woods DF, Bryant PJ (1991) The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 66, 451–64.
Yanai H, Satoh K, Matsumine A, Akiyama T (2000) The colorectal tumour suppressor APC is present in the NMDA-receptor-PSD-95 complex in the brain. Genes Cells 5, 815–22.
Zhang F, White RL, Neufeld KL (2000) Phosphorylation near nuclear localization signal regulates nuclear import of adenomatous polyposis coli protein. Proc Natl Acad Sci USA 97, 12 577–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senda, T., Shimomura, A. & Iizuka-Kogo, A. Adenomatous polyposis coli (Apc) tumor suppressor gene as a multifunctional gene. Anato Sci Int 80, 121–131 (2005). https://doi.org/10.1111/j.1447-073x.2005.00106.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1447-073x.2005.00106.x