Abstract
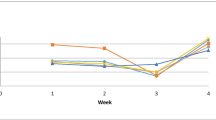
Effects of probiotics on growth, stress tolerance and non-specific immune response in Japanese flounder Paralichthys olivaceus were evaluated in a closed recirculating system. Survival and growth of flounder treated by supplying commercial probiotics either in the diet (the probiotic diet group), or into the rearing water (the water supply group), were higher compared to the untreated group (the control group). Water quality parameters, pH, NH4−N, NO2−N and PO4−P showed lower concentration in the probiotic diet group compared with the control group and the supply group. Plasma lysozyme activity in the probiotic diet group and the water supply group was significantly higher (P<0.05) than that in the control group. In heat shock stress tests, flounder in the probiotics-treated groups showed greater heat tolerance (measured by 50% lethal time, LT50) than the control group. Pathogen challenge tests with Vibrio anguillarum (2×107 c.f.u./mL) resulted in significantly higher survival in the probiotics-treated groups than the control group. Results indicated that probiotics supplied in the rearing water and the diet of fish enhanced the stress tolerance and the non-specific immune system of Japanese flounder, providing them a higher resistance against stress conditions and pathogens.
Similar content being viewed by others
References
Olafsen JA. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 2001; 200: 223–247.
Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. 1989; 66: 365–378.
Salminen S, Isolauri E, Salminen E. Clinical uses of probiotics for stabilizing the gut mucosal barrier: successful strains and future challenge. Antonie van leeuuwenboek 1997; 70: 347–358.
Sakai M. Current research status of fish immunostimulants. Aquaculture 1998; 172: 63–92.
Corre V Jr, Janeo RJ, Caipang AMA. Use of probiotics, reservoir with green water in shrimp farming in the Philippines. Proceed. Jpn. Soc. Promotion Sci. (JSPS) 2001; 30: 67–72.
Moriarty DJW. Control of luminous Vibrio species in penaeid aquaculture pond. Aquaculture 1998; 164: 351–358.
Nogami K, Hamasaki K, Maeda M, Hirayama K. Biocontrol method in aquaculture for rearing the swimming crab larvae Portunus trituberculatus. Hydrobiologia 1997; 358: 291–295.
Irianto A, Austin B. Probiotics in aquaculture. J. Fish. Dis. 2002; 25: 633–642.
Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture 1998; 167: 301–313.
Riquelme C, Araya R, Nelson V, Rojas A, Guaita M, Candia M. Potential probiotic strains in the culture of the Chilean scallop Argopecten purratus (Lamarck, 1819). Aquaculture 1997; 154: 17–26.
Byun JW. Probiotic effect of Lactobacillus sp. Ds-12 in flounder. J. Gen. Appl. Microbiol. 1997; 43: 305–308.
Robertson PAW, O’Dowd C, Burrells C, Williams P, Austin B. Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorynchus mykiss, Walbaum). Aquaculture 2000; 185: 235–243.
Gatescope FJ. Lactic acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic Vibrio. Aquat. Living Resour. 1994; 7: 277–282.
Sugita H, Tanaami H, Kobashi, T, Geguchi Y. Bacterial flora of coastal bivalves. Nippon Suisan Gakkaishi 1981 47: 655–661.
Yamanoi H, Muroga K, Maruyama K. A bacteriological investigation on the mass mortalities of red seabream Pagrus major larvae with intestinal swelling. Suisanzoshoku 1998; 36: 11–20.
Aranishi F, Nakane M. Epidermal protease of the Japanese eel. Fish Physiol. Biochem. 1997; 16: 471–478.
Newell B, Morgan B, Cundy J. Determination of Urea in seawater. J. Mar. Res. 1967; 25: 201–202.
Strickland JDH, Parsons TA. Practical Hand Book of Seawater Analysis. Bull. Fish. Res. Ed. Can. 1972; 167: 1–311.
Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th edn. Association of Official Analytical Chemists, Arlington, VA. 1995.
Lowly OH, Rosebrough NJ, Fan AC, Rondall RT. Protein measurement with the folin phenol solution. J. Biol. Chem. 1951; 193: 265.
Takahashi Y, Itami T, Konegawa K. Enzymatic properties of partially lysozyme from the skin mucus of carp. Nippon Suisan Gakkaishi 1986; 52: 1209–1214.
Caruso D, Schlumberger O, Dahm C, Proteau JP. Plasma lysozyme levels in sheatfish Silurus glanis (L) subjected to stress and experimental infection with Edwardsiella tarda. Aquacult. Res. 2002; 33: 999–1008.
Koshio S, Sakakura Y, Iida Y, Tsukamoto K, Kida T, Darbrowsky K. The effect of vitamin C intake on schooling behavior of amphidromus fish, Ayu Plecoglossus altivelis. Fish. Sci. 1997; 63: 619–624.
Austin B, Stuckey LF, Robertson PAW, Effendi I, Griffith DRW. A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonous salmonicida, Vibrio anguillarum and Vibrio ordalii. J. Fish Dis. 1995; 18: 93–96.
Chen CC, Chen SN. Water quality management with Bacillus spp. in the high-density culture of red-parrot fish Cichlasona citrinellum x C. sunspilum. North Am. J. Aquac. 2001; 63: 66–73.
De Schrijver R, Ollevier F. Protein digestion in juvenile turbot (Scophthalmus maximus) and effects of dietary administration of Vibrio proteolyticus. Aquaculture 2000; 186: 107–116.
Konsula Z, Liakopoulou-Kyriakides M. Hydrolysis of starches by the action of an α-amylase from Bacillus subtilis. Process Biochem. 2004; 39: 1745–1749.
Ben Messaound E, Ben Ali M, Elleuch N, Fourati Masmoudi N, Bejar S. Purification and properties of a malthoheptaoseand maltohexaose forming amylase produced by Bacillus subtilis US116. Enzyme Microb. Technol. 2004; 34: 662–666.
Ellouz Y, Bayoudh A, Kammoun S, Charsallah N, Nasri M. Productions of protease by Bacillus subtilis grown on sardinell heads and viscera. Bioresource Technol. 2001; 80; 49–51.
Ellis AE. Techniques in fish immunology. In: Stolen JS, Fletcher TC, Anderson DP, Robertson BS, Van Muiswinkle WB (eds). Lysozyme Assays. SCS Publications, Fair Haven. 1990; 101–103.
Grinde B. Lysozyme from rainbow trout Salmo gairdneri Richardson as an anti bacterial agent against fish pathogens. J. Fish Dis. 1989; 12: 207–210.
Haijji N, Sugita H, Ishii S, Deguchi Y. Serum bactericidal activity of carp (Cyprinus carpio) under supposed stressful rearing conditions. Bull. Coll. Agric. Vet. Med., Nihon Univ. 1990; 47: 50–54.
Mock A, Peters GS. Lysozyme activity in rainbow trout Oncorynchus mykiss (Walbaum) stressed by handling transport and water pollution. J. Fish Biol. 1990; 37: 873–885.
Caruso D, Lazard J. Subordination stress in Nile tilapia and its effect on plasma lysozyme activity. J. Fish. Biol. 1999; 55: 451–454.
Yehuda R, Yang R.-K, Guo SL, Makokine I, Singh B. Relationship between dexamethasone-inhibited lysozyme activity in peripheral mononuclear leukocytes and the cortisol and glucocorticoid receptor response to dexamethasone. J. Psychiatric Res. 2003; 37: 471–477.
Demers NE, Bayne CJ. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997; 21: 363–373.
Kakuta I, Hisashi K, Nakamura H, Yamauchi K. Enhancement of the nonspecific defense activity of the skin mucus of red sea bream by oral administration of bovine lactoferin. Suisanzoshoku 1996; 44: 197–202.
Weyts FAA, Cohon N, Flik G, Verburg-Van Kemehande BMK. Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish. Fish Shellfish Immunol. 1999; 9: 1–20.
Joborn A, Olsson JC, Westerdahl A, Conway PL, Kjelleberg S. Colonization in the fish intestinal tract and production of inhibitory substances in intestinal mucus and faecal extracts by Carnobacterium sp. strain K1. J. Fish Dis. 1997; 20: 383–392.
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P. Immunity enhancement in black tiger shirimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 2000; 191: 271–288.
Nikoskelainen S, Ouwehand A, Salminen S, Bylund G. Protection of rainbow trout (Oncorynchus myliss) from frunculosis by Lactobacillus rhamnosus. Aquaculture 2001; 198: 229–236.
Gildberg A, Mikkelsen H. Effects of supplementing the feed to Atlantic cod (Gadus morhua) fry with lactic acid bacteria and immuno-stimulating peptides during a challenge trial with Vibrio anguillarum. Aquaculture 1998; 167: 103–113.
Gildberg A, Johansen A, Bogwald J. Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquaculture 1995; 138: 23–34.
Fevolden SE, Refstie T, Roed KH. Disease resistance in rainbow trout (Oncorhynchus mykiss) selected for stress response. Aquaculture 1992; 104: 19–29.
Ringo E, Birkbeck TH, Munro DO, Vadstein O, Hielmeland K. The effect of early exposure to Vibrio pelagius on the aerobic bacterial flora of turbot Scophthalmus maximus (L) larvae. J. Appl. Bacteriol. 1996; 81: 207–211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taoka, Y., Maeda, H., Jo, JY. et al. Growth, stress tolerance and non-specific immune response of Japanese flounder Paralichthys olivaceus to probiotics in a closed recirculating system. Fish Sci 72, 310–321 (2006). https://doi.org/10.1111/j.1444-2906.2006.01152.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1444-2906.2006.01152.x