Summary
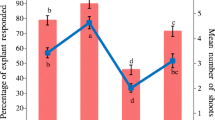
We have developed a highly efficient two-stage protocol for induction of multiple shoots from single node in vitro shoot tip explants of Decalepis hamiltonii. It was found that phenylacetic acid (PAA) had a synergistic effect on shoot multiplication when treated with N6-benzyladenine (BA). This protocol used PAA for both multiple shoot induction from nodal explants, elongation of primary shoots, and initiation of adventitious shoot formation from primary shoots. Murashige and Skoog medium containing BA (2.22–31.08 μM) and α-naphthaleneacetic acid (0.27–10.74 μM) or PAA (7.34–36.71 μM) was used to initiate shoot formation from nodal explants. The maximum number of shoots per culture was produced on a medium containing 31.08 μM BA and 14.68 μM PAA, while the longest shoot length and nodes were obtained on medium containing 22.2 μM BA and 14.68 μM PAA. Shoots subcultured on MS medium containing 22.2 μM BA and 14.68 μM PAA elongated along with secondary shoot formation. The shoots were rooted on medium containing 9.7 μM indole-3-butyric acid. The plantlets were acclimatized in soil with an 80–90% survival rate under field conditions.
Similar content being viewed by others
References
Abe, H.; Uchimiya, M.; Sato, R. Isolation of phenyl acetic acid and its p-hydroxy derivative as auxin like substances from Undaria pinnatifida. Agric. Biol. Chem. 38:897–898; 1974.
Degelene, L.; Lesignes, P.; Alibert, G.; Sarrafi, A. Genetic control of organogenesis in cotyledons of sunflower (Helianthus annuus). Plant Cell Tiss. Organ Cult. 48:127–130; 1997.
George, J.; Bais, H. P.; Ravishankar, G. A.; Manilal, P. Optimization of media constituents for shoot regeneration from leaf callus culture of Decalepis hamiltonii Wight & Arn. HortScience 35:296–299; 2000.
George, J.; Pereira, J.; Divakar, S.; Udaysankar, K.; Ravishankar, G. A. A method for preparation of active fraction from the root of Decalepis hamiltonii, useful as bioinsecticides. (Indian patent no. 1301/Del/98); 1998.
Hussain, S.; Jain, A.; Kothari, S. L. Phenylacetic acid improves bud elongation and in vitro plant regeneration efficiency in Capsicum annum L. Plant Cell Rep. 19:64–68; 1999.
Isogai, Y.; Okomoto, T.; Koizumi, T. Plant growth regulators. Isolation of indole-3-acetamide, 2-phenyl-acetamide and indole-3-carboxaldehyde from etiolated seedlings of Phaseolus. Chem. Pharm. Bull. (Tokyo) 15:151–158; 1967.
Jethwani, V.; Kothari, S. L. Phenyl acetic acid induced organogenesis in cultured leaf segments of Dianthus chinensis. Plant Cell Rep. 15:869–872; 1996.
Knittel, N.; Escandon, A. S.; Hahne, G. Plant regeneration at high frequency from mature sunflower cotyledons. Plant Sci. 73:219–226; 1991.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Phadke, N. Y.; Gholap, A. S.; Subbalakshmi, G.; Ramakrishnan, K. Essential oil of Decalepis hamiltonii as an antimicrobial agent. J. Food Sci. Technol. 31:472–475; 1994.
Pua, E. C.; Sim, G. E.; Chi, G. L.; Kong, L. F. Synergistic effects of ethylene inhibitors and putrescine on shoot regeneration from hypocotyl explants of Chinese radish (Raphanus sativus L. var. longipinnatus Bailey) in vitro. Plant Cell Rep. 15:685–690; 1996.
Reddy, B. O.; Giridhar, P.; Ravishankar, G. A. In vitro propagation of Decalepis hamiltonii Wight & Arn., an endangered shrub by auxins and root promoting agents. Curr. Sci. 81:1479–1482; 2001.
Schneider, E. A.; Whightman, F. Auxins on non-flowering plants. Occurrence of 3-indole acetic acid and phenyl acetic acid in vegetative and fertile fronds of the stritch fern (Matteucia struthiopteris). Physiol. Plant. 68:396–402; 1986.
Slimmon, T.; Qureshi, J. A.; Saxena, P. K. Phenylacetic acid induced somatic embryogenesis in cultured hypocotyl explants of Geranium (Pelargonium × hortorum Bailey). Plant Cell Rep. 10:587–589; 1991.
Small, D. K.; Morris, D. A. Promotion of elongation and acid invertase activity in Phaseolus vulgaris L. internode segments by phenylacetic acid. Plant Growth Regul. 9:329–340; 1990.
Wealth of India. Decalepis hamiltonii Wight & Arn. In: Raw materials, vol. 3. New Delhi, India; CSIR; 1990:161–162.
Wheeler, A. Auxin like growth activity of phenylacetonitrile. Ann. Bot. 41:867–872; 1977.
Whightman, F.; Schneider, E. A.; Thimann, K. V. Hormonal factors controlling the initiation and development of lateral roots and effects of exogenous growth factors on lateral root formation in pea roots. Physiol. Plant. 49:304–314; 1980.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giridhar, P., Ramu, D.V., Reddy, B.O. et al. Influence of phenylacetic acid on clonal propagation of Decalepis hamiltonii wight & ARN: An endangered shrub. In Vitro Cell.Dev.Biol.-Plant 39, 463–467 (2003). https://doi.org/10.1079/IVP2003448
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2003448