Summary
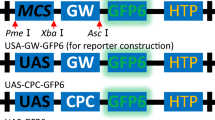
The ability to create artificial gene-clusters for genetic transformation could facilitate the development of crops with multiple engineered traist, or with traits which result from the expression of multiple genes. A simple method to assemble artificial gene-clusters was developed by designing a multiple cloning site consisting of an array of homing endonuclease cleavage sites into a single vector. These enzymes are also known as intron-or intein-encoded endonucleases, and have very long recognition sequences, which makes them very rare cutters. The resulting vectors are pUGA for microprojectile-mediated transformation, and pUGA2 for Agrobacterium-mediated transformation. In addition, a series of unidirectional shuttle vectors containing various combinations of homing endonuclease restriction sites was constructed. Gene cassettes can be cloned into individual shuttles, and then transferred to either pUGA or pUGA2 to construct artificial gene-clusters. To test the feasibility of this approach, a six-gene cluster was constructed and transformed into soybean via microprojectile bombardment and into tobacco via Agrobacterium. The genes were assayed for expression in both the T0 and T1 generations for three independent transgenics. Up to five of the six genes were expressed. Additional changes to the construction of individual gene cassettes may improve the frequency with which all genes in the cluster are expressed.
Similar content being viewed by others
References
Allen, G. C.; Spiker, S.; Thompson, W. F. Use of matrix attachment regions (MARd) to minimize transgene silencing. Plant Mol. Biol. 43:361–376; 2000.
An, Y.-Q.; McDowell, J. M.; Huang, S.; McKinney, E. C.; Chambliss, S.; Meagher, R. B. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10:107–121; 1996.
Asselberg, F. A. M.; Rival, S. Creation of a novel, versatile multiple cloning site cut by four rare-cutting homing endonucleases. BioTechniques 20:558–562; 1996.
Becker, D.; Kemper, E.; Schell, J.; Masterson, R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol. Biol. 20:1195–1197; 1992.
Belfort, M.; Raeban, M. E.; Coetzee, T.; Dalgaard, J. Z. Prokaryotic introns and inteins: a panoply of form and function. J. Bacteriol. 177:3897–3903; 1995.
Bower, R.; Elliott, A. R.; Potier, B. A. M.; Birch, R. G. High-efficiency, microprojectile-mediated cotransformation of sugarcane, using visible or selectable markers. Mol. Breed. 2:239–249; 1996.
Breyne, P.; Gheysen, G.; Jacobs, A.; Van Montagu, M.; Depicker, A.. Effect of T-DNA configuration on transgene expression. Mol. Gen. Genet. 235:389–396; 1992.
Campbell, B. T.; Baenziger, P. S.; Mitra, A.; Sato, S.; Clemente, T. Inheritance of multiple transgenes in wheat. Crop. Sci. 40:1133–1141; 2000.
Chen, L.; Marmey, P.; Taylor, N. J.; Brizard, J.-P.; Espinoza, C.; D'Cruz, P.; Huet, H.; Zhang, S.; de Kochko, A.; Beachy, R. N.; Fauquet, C. M. Expression and inheritance of multiple transgenes in rice plants. Nat. Biotechnol. 16:1060–1064; 1998.
Chomczynski, P. One-hour alkaline capillary transfer for blotting of DNA and RNA. Anal. Biochem. 201:134–139; 1992.
Colleaux, L.; d'Auriol, L.; Betermier, M.; Cottarel, G.; Jacquier, A.; Gilbert, F.; Dujon, B. Universal code equivalent of a yeast mitochondrial intron reading frame is expressed in E. coli as a specific double strand endonuclease. Cell 44:521–533; 1986.
Colston, M. J.; Davis, E. O. The ins and outs of protein splicing elements. Mol. Microbiol. 12:359–363; 1994.
Davis, S. J.; Vierstra, R. D. Soluble, highly fluorescent variants of green fluorescent protein (GFP) for use in higher plants. Plant Mol. Biol. 36:521–528; 1998.
Garbarino, J. E.; Belknap, W. R. Isolation of a ubiquitin-ribosomal protein gene (ubi3) from potato and expression of its promoter in transgenic plants. Plant Mol. Biol. 24:119–127; 1994.
Hajdukiewicz, P.; Svab, Z.; Maliga, P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25:989–994; 1994.
Halpin, C.; Barakate, A.; Askari, B. M.; Abbott, J. C.; Ryan, M. D. Enabling technologies for manipulating multiple genes on complex pathways. Plant Mol. Biol. 47:295–310; 2001.
Hamilton, C. M.; Frary, A.; Lewis, C.; Tanksley, S. D. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl Acad. Sci. USA 93:9975–9979; 1996.
Hunt, A. C.; Maiti, I. B. Strategies for expressing multiple foreign genes in plants as polycistronic constructs. In Vitro Cell. Dev. Biol. Plant 37:313–320; 2001.
Ingelbrecht, I.; Breyne, P.; Vanecompernolle, K.; Van Montagu, M.; Depicker, A. Transcriptional interference in transgenic plants. Gene 109:239–242;1991.
Jefferson, R. A. The GUS reporter gene system. Nature 342:837–838; 1989.
Komari, T.; Hiei, Y.; Saito, Y.; Murai, N.; Kumashiro, T. Vectors carrying two separate T-DNAs for cotransformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J. 10:165–174; 1996.
Kramer, C.; DiMaio, J.; Carswell, G. K.; Shillito, R. D. Selection of transformed protoplast-derived Zea mays colonies with phosphinothricin and a novel assay using the pH indicator chlorophenol red. Plant Cell Rep. 190:454–458; 2002.
Lambowitz, A. M.; Belfort, M. Innous as mobile genetic elements. Annu. Rev. Biochem. 62:587–622; 1993.
Lebel, E. G.; Masson, J.; Bogucki, A.; Paszkowski, J. Transposable elements as plant transformation vectors for long stretches of foreign DNA. Theor. Appl. Genet. 91:899–906; 1995.
Lemieux, C.; Turmel, M. A group I intron in the chloroplast large subunit rRNA gene of Chlamydomonas eugametos encodes a double-strand endonuclease that cleaves the homing site of this intron. Curr. Gen. 19:43–47; 1991.
Mueller, J. E.; Bryk, M.; Lozios, N.; Belfort, M. Homing endonucleases. In: Linn, S. M.; Lloyd, R. S.; Roberts, R. J., eds. Nucleases, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993:111–143.
Mullen, J. A.; Adam, G.; Blowers, A.; Earle, E. D. Biolistic transfer of large DNA fragments to tobacco cells using YACs retrofitted for plant transformation. Mol. Breed. 4:449–457; 1998.
Muscerella, D. E.; Vogt, V. M. A mobile group I intron in the nuclear rDNA of Physarum polycephalum. Cell 56:443–454; 1989.
Nakashita, H.; Arai, Y.; Shikanai, T.; Doi, Y.; Yamaguchi, I. Introduction of bacterial metabolism into higher plants by polycistronic transgene expression. Biosci. Biotechnol. Biochem. 65:1688–1691; 2001.
Padidam, M.; Cao, Y. J. Elimination of transcriptional interference between tandem genes in plant cells. Bio Techniques 31:328–334; 2001.
Perler, F. B.; Comb, D. G.; Jack, W. E.; Moran, L. S.; Qiang, B.; Kucera, R. B.; Benner, J.; Slatko, B. E.; Nwankwo, D. O.; Hempstead, S. K.; Carlow, C. K. S.; Jannasch, H. Intervening sequences in an Archea DNA polymerase gene. Proc. Natl Acad. Sci. USA 89:5577–5581; 1992.
Perler, F. B.; Davis, E. O.; Dean, G. E.; Gimble, F. S.; Jack, W. E.; Neff, N.; Noren, C. J.; Thorner, J.; Belfort, M. Protein splicing elements: inteins and exteins—a definition of terms and recommended nomenelature. Nucl. Acids Res. 22:1125–1127; 1994.
Sambrook, J.; Fritsch, E. F.; Maniatis, T. Molecular cloning. A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
Schardl, C. L.; Byrd, A. D.; Benzion, G.; Altschuler, M. A.; Hildebrand, D. F.; Hunt, A. G. Design and construction of a versatile system for the expression of foreign genes in plants. Gene 61:1–11; 1987.
Schmidt, M. A.; Parrott, W. A. Quantitative detection of transgenes in soyben [Glycine max (L.) Mirrill] and peanut (Arachis hypogaea L.) by rel-time polymerase chain reaction. Plant Cell Rep. 20:422–428; 2001.
Shibata, D.; Liu, Y.-G. Agrobacterium-mediated plant transformation with large DNA fragments. Trends Plant Sci. 5:354–355; 2000.
Sugita, K.; Matsumaga, E.; Kasahara, T.; Ebinuma, H. Transgene stacking in plants in the absence of sexual crossing. Mol. Breed. 6:529–536; 2000.
Thomson, J. M.; Compton, M. M. Disposable device for the isolation of DNA from agarose gels. BioTechniques 24:942; 1998.
Thomson, J. M.; Parrott, W. A. pMECA: a cloning plasmid with 44 unique restriction sites that allows selection of recombinants based on colony size. Bio Techniques 24:922–927; 1998.
Trick, H. N.; Dinkins, R. D.; Santarem, E. R.; Di, R.; Samoylov, V. M.; Meurer, C.; Walker, D.; Parrott, W. A.; Finer, J. J.; Collins, G. B. Recent advances in soybean transformation. Plant Tiss. Cult. Biotechnol. 3:9–26; 1997.
Überlacker, B.; Werr, W. Vectors with rate-cutter restriction enzyme sites for expression of open reading frames in trasgenic plants. Mol. Breed. 2:293–295; 1996.
Xu, M. Q.; Southworth, M. W.; Mersha, F. B.; Hornstra, L. J.; Perler, F. In vitro protein splicing of a purified precursor and the identification of a branched intermediate. Cell 75:1371–1377; 1993.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michael Thomson, J., Lafayette, P.R., Schmidt, M.A. et al. Artificial gene-clusters engineered into plants using a vector system based on intron-and intein-encoded endonucleases. In Vitro Cell.Dev.Biol.-Plant 38, 537–542 (2002). https://doi.org/10.1079/IVP2002329
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1079/IVP2002329