Abstract
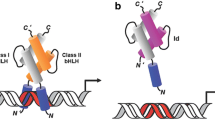
The Id (inhibitor of differentiation or DNA binding) helix-loop-helix (HLH) proteins are a group of dominant negative regulators of basic HLH transcriptional factors which promote cell differentiation. Recent evidence has revealed that Id proteins, especially Id-1, are also able to promote cell proliferation and cell cycle progression through inactivation of tumour suppressor and activation of growth promoting pathways in mammalian cells. In addition, upregulation of Id-1 has been found in many types of human cancer and its expression levels are also associated with advanced tumour stage. Furthermore, ectopic expression of Id-1 in human cancer cells is able to induce cell proliferation under sub-optimal conditions and protect the cells against apoptosis. These lines of evidence strongly indicate Id-1 as a positive regulator of cell growth and its expression may be a key factor required for tumour cell proliferation. This review will discuss recent evidence on the role of Id-1 in cell proliferation and survival, and its significance in malignant transformation. In addition, we will highlight the recent development in the understanding of the molecular mechanisms responsible for the action of Id-1 in promoting cell survival and tumourigenesis. Finally, the therapeutic implications through inactivation of Id-1 in the treatment of human cancer will also be addressed.
Similar content being viewed by others
References
Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ 1993; 4: 49–55.
Olson, EN, Klein WH. bHLH factors in muscle development: dead line and commitments, what to leave in and what to leave out. Genes Dev 1994; 8: 1–8.
Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell 1990; 61: 49–59.
Norton JD, Deed RW, Craggs, G, Sablitzky F. Id helix-loop-helix proteins in cell growth and differentiation. Trends Cell Biol 1998; 8: 58–65.
Evans SM, O’Brien TX. Expression of the helix-loop-helix factor Id during mouse embryonic development. Dev Biol 1993; 159: 485–499.
Riechmann V, Sablitzky F. Mutually exclusive expression two dominant-negative helix-loop-helix (dnHLH) genes, Id4 and Id3, in the developing brain of the mouse suggests distinct regulatory roles of these dnHLH proteins during cellular proliferation and differentiation of the nervous system. Cell Growth Differ 1995; 6: 837–843.
Yokota Y. Id and development. Oncogene 2001; 20: 8290–8298.
Lyden D, Young AZ, Zagzag D, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 1990; 401: 670–677.
Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37: 614–636.
Alani RM, Hasskarl J, Grace M, Hernandez MC, Israel MA, Munger K. Immortalization of primary human keratinocytes by the helix-loop-helix protein, Id-1. Proc Natl Acad Sci USA 1999; 96: 9637–9641.
Nickoloff BJ, Chaturvedi V, Bacon P, Qin JZ, Denning MF, Diaz MO. Id-1 delays senescence but does not immortalize keratinocytes. J Biol Chem 2000; 275: 27501–27504.
Foreman KE, Friborg J, Chandran B, et al. Injection of human herpesvirus-8 in human skin engrafted on SCID mice induces Kaposi’s sarcoma-like lesions. J Dermatol Sci 2001; 26: 182–193.
Tang J, Gordon GM, Muller MG, Dahiya M, Foreman KE. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J Virol 2003; 77: 5975–5984.
Ouyang XS, Wang X, Lee DT, Tsao SW, Wong YC. Over expression of ID-1 in prostate cancer. J Urol 2002; 167: 2598–2602.
Lin CQ, Singh J, Murata K, et al. A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Res 2000; 60: 1332–1340.
Schindl M, Oberhuber G, Obermair A, Schoppmann SF, Karner B, Birner P. Overexpression of Id-1 protein is a marker for unfavorable prognosis in early-stage cervical cancer. Cancer Res 2001; 61: 5703–5706.
Schindl M, Schoppmann SF, Strobel T, et al. Level of Id-1 protein expression correlates with poor differentiation, enhanced malignant potential, and more aggressive clinical behavior of epithelial ovarian tumors. Clin Cancer Res 2003; 9: 779–785.
Schoppman SF, Schindl M, Bayer G, et al. Overexpression of Id-1 is associated with poor clinical outcome in node negative breast cancer. Int J Cancer 2003; 104: 677–682.
Volpert OV, Pili R, Sikder HA, et al. Id1 regulates angiogenesis through transcriptional repression of thrombospondin-1. Cancer Cell 2002; 2: 473–483.
Wice BM, Gordon JI. Forced expression of Id-1 in the adult mouse small intestinal epithelium is associated with development of adenomas. J Biol Chem 1998; 273: 25310–25319.
Kreider BL, Benezra R, Rovera G, Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science 1992; 255: 1700–1702.
Shoji W, Yamamoto T, Obinata M. The helix-loop-helix protein Id inhibits differentiation of murine erythroleukemia cells. J Biol Chem 1994; 269: 5078–5084.
Lister J, Forrester WC, Baron MH. Inhibition of an erythroid differentiation switch by the helix-loop-helix protein Id1. J Biol Chem 1995; 270: 17939–17946.
Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev 1992; 6: 1466–1479.
Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells 2002; 7: 949–960.
Ling MT, Wang X, Tsao SW, Wong YC. Down-regulation of Id-1 expression is associated with TGF beta 1–induced growth arrest in prostate epithelial cells. Biochim Biophys Acta 2002; 1570: 145–152.
Ma Y, Koza-Taylor PH, DiMattia DA, et al. Microarray analysis uncovers retinoid targets in human bronchial epithelial cells. Oncogene 2003; 22: 4924–4932.
Goldstein S. Replicative senescence: The human fibroblast comes of age. Science 1990; 249: 1129–1133.
McConnell BB, Starborg M, Brookes S, Peters G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol 1998; 8: 351–354.
Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol 1996; 16: 859–867.
Alani RM, Young AZ, Shifflett CB. Id1 regulation of cellular senescence through transcriptional repression of p16/Ink4a. Proc Natl Acad Sci USA 2001; 98: 7812–7816.
Tang J, Gordon GM, Nickoloff BJ, Foreman KE. The helix-loop-helix proteni id-1 delays onset of replicative senescence in human endothelial cells. Lab Invest 2002; 82: 1073–1079.
Hara E, Uzman JA, Dimri GP, Nehlin JO, Testori A, Campisi J. The helix-loop-helix protein Id-1 and a retinoblastoma protein binding mutant of SV40 T antigen synergize to reactivate DNA synthesis in senescent human fibroblasts. Dev Genet 1996; 18: 161–172.
Norton JD, Atherton GT. Coupling of cell growth control and apoptosis functions of Id proteins. Mol Cell Biol 1998; 18: 2371–2381.
Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345: 458–460.
Ohtani N, Zebedee Z, Huto TJ, et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 2001; 409: 1067–1070.
Sherr CJ. Cancer cell cycles. Science 1996; 274: 1672–1677.
Tournay O, Benezra R. Transcription of the dominant-negative helix-loop-helix protein Id1 is regulated by a protein complex containing the immediate-early response gene Egr-1. Mol Cell Biol 1996; 16: 2418–2430.
Schiffman MH, Brinton LA. The epidemiology of cervical carcinogenesis. Cancer 1995; 76: 1888–1901.
Tsao SW, Wong N, Wang X, et al. Nonrandom chromosomal imbalances in human ovarian surface epithelial cells immortalized by HPV16–E6E7 viral oncogenes. Cancer Genet Cytogenet 2001; 130: 141–149.
Vandeputte DA, Troost D, Leenstra S, et al. Expression and distribution of id helix-loop-helix proteins in human astrocytic tumors. Glia 2002; 38: 329–338.
Chaturvedi V, Bonish B, Bacon P, et al. Role for Id-1 in immunobiology of normal keratinocytes and in basal cell carcinoma. Exp Dermatol 2003; 12: 255–260.
Wilson JW, Deed RW, Inoue T, et al. Expression of Id helix-loop-helix proteins in colorectal adenocarcinoma correlates with p53 expression and mitotic index. Cancer Res 2001; 61: 8803–8810.
Takai N, Miyazaki T, Fujisawa K, Nasu K, Miykawa I. Id1 expression is associated with histological grade and invasive behavior in endometrial carcinoma. Cancer Lett 2001; 165: 185–193.
Hu YC, Lam KY, Law S, Wong J, Srivastava G. Identification of differentially expressed genes in esophageal squamous cell carcinoma (ESCC) by cDNA expression array: Overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC. Clin Cancer Res 2001; 7: 2213–2221.
Langlands K, Down GA, Kealey T. Id proteins are dynamically expressed in normal epidermis and dysregulated in squamous cell carcinoma. Cancer Res 2000; 60: 5929–5933.
Lee TK, Man K, Ling MT, et al. Over-expression of Id-1 induces cell proliferation in hepatocellular carcinoma through inactivation of p16INK4a/RB pathway. Carcinogenesis 2003; 24: 1729–1736.
Polsky D, Young AZ, Busam KJ, Alani RM. The transcriptional repressor of p16/Ink4a, Id1, is up-regulated in early melanomas. Cancer Res 2001; 61: 6008–6011.
Wang X, Xu K, Ling MT, et al. Evidence of increased Id-1 expression and its role in cell proliferation in nasopharyngeal carcinoma cells. Mol Carcinog 2002; 35: 42–49.
Nishimine M, Nakamura M. Mishima K, et al. Id proteins are overexpressed in human oral squamous cell carcinomas. J Oral Pathol Med 2003; 32: 350–357.
Maruyama H, Kleeff J, Wildi S, et al. Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am J Pathol 1999; 155: 815–822.
Sablitzky F, Moore A, Bromley M, Deed RW, Newton JS, Norton JD. Stage-and subcellular-specific expression of Id proteins in male germ and Sertoli cells implicates distinctive regulatory roles for Id proteins during meiosis, spermatogenesis, and Sertoli cell function. Cell Growth Differ 1998; 9: 1015–1024.
Kebebew E, Treseler PA, Duh QY, Clark OH. The helix-loop-helix transcription factor, Id-1, is overexpressed in medullary thyroid cancer. Surgery 2000; 128: 952–957.
Kebebew E, Treseler PA, Duh QY, Clark OH. The helix-loop-helix protein, Id-1, is overexpressed and regulates growth in papillary thyroid cancer. Surgery 2003; 134: 235–241.
Ouyang XS, Wang X, Ling MT, Wong HL, Tsao SW, Wong YC. Id-1 stimulates serum independent prostate cancer cell proliferation through inactivation of p16(INK4a)/pRB pathway. Carcinogenesis 2002; 23: 721–725.
Desprez PY, Lin CQ, Thomasset N, Sympson CJ, Bissell MJ, Campisi J. A novel pathway for mammary epithelial cell invasion induced by the helix-loop-helix protein Id-1. Mol Cell Biol 1998; 18: 4577–4588.
Fong S, Itahana Y, Sumida T, et al. Id-1 as a molecular target in therapy for breast cancer cell invasion and metastasis. Proc Natl Acad Sci USA 2003; 100: 13543–13548.
Ling MT, Wang X, Ouyang XS, Xu K. Tsao SW, Wong YC. Id-1 expression promotes cell survival through activation of NF-kappaB signalling pathway in prostate cancer cells. Oncogene 2003; 22: 4498–4508.
Ling MT, Wang X, Tam PC, Tsao SW, Wong YC. Id-1 expression induced androgen independent prostate cancer cell growth through activation of epidermal growth factor receptor (EGF-R). Carcinogenesis 2003.
Ling MT, Wang X, Ouyang XS, et al. Activation of MAPK signaling pathway is essential for Id-1 induced serum independent prostate cancer cell growth. Oncogene 2002; 21: 8498–8505.
Tanaka K, Pracyk JB, Takeda K, et al. Expression of Id1 results in apoptosis of cardiac myocytes through a redox-dependent mechanism. J Biol Chem 1998; 273; 25922–25928.
Florio M, Hernandez MC, Yang H, Shu HK, Cleveland JL, Israel MA. Id2 promotes apoptosis by a novel mechanism independent of dimerization to basic helix-loop-helix factors. Mol Cell Biol 1998; 18: 5435–5444.
Kim D, Peng XC, Sun XH. Massive apoptosis of thymocytes in T-cell-deficient Id1 transgenic mice. Mol Cell Biol 1999; 19: 8240–8253.
Parrinello S, Lin CQ, Murata K, et al. Id-1, ITF-2, and Id-2 comprise a network of helix-loop-helix proteins that regulate mammary epithelial cell proliferation, differentiation, and apoptosis. J Biol Chem 2001; 276; 39213–39219.
Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 2001; 7: 1194–1201.
de Candia P, Solit DB, Giri D, et al. Angiogenesis impairment in Id-deficient mice cooperates with an Hsp90 inhibitor to completely suppress HER 2/neu-dependent breast tumors. Proc Nal Acad Sci USA 2003; 100: 12337–12342.
Ruzinova MB, Schoer RA, Gerald W, et al. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell 2003; 4: 277–289.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, YC., Wang, X. & Ling, MT. Id-1 expression and cell survival. Apoptosis 9, 279–289 (2004). https://doi.org/10.1023/B:APPT.0000025804.25396.79
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000025804.25396.79