Abstract
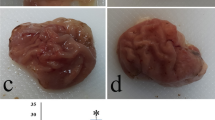
The mucosal protective effect of nitric oxide(NO) was examined by usingNG-nitro-L-arginine methyl ester (L-NAME) asnitric oxide synthase (NOS) inhibitor and nitroprusside(NP) as NO donating agent, in ethanol-induced rat gastric lesion model.The results are summarized as follows: (1) As gastrictissue samples were examined by light microscopy,intragastric exposure of ethanol was demonstrated to induce gastric injury, which was more prominentin female rats. The depletion of NO by L-NAME treatmentexacerbated the ethanol-induced gastric lesion but NPtogether with ethanol promoted repair of the mucosal injury, especially in female rats. (2)Gastric H+, K+-ATPase enzymeactivity, which was responsible for acid secretion,seemed not to be effected by ethanol treatment. Togetherwith ethanol, L-NAME treatment activated, whereas NP treatmentinhibited, the enzyme activity in female rats. (3)Ethanol treatment inhibited gastric alcoholdehydrogenase (ADH) activity, which was responsible forthe first-pass metabolism of ethanol. Together with ethanol,L-NAME did not effect the enzyme activity whereas NPtreatment disappeared the inhibitory effect of ethanolin both gender. Hydroxyl radical (OH•) scavenger activity was found to increase in ethanol andethanol + NP groups in both sexes, but superoxideradical (O2 -•) scavengeractivity did not change. The results indicate that NOmay ameliorate the damaging effect of ethanol possibly by regulating acidsecretion, ethanol metabolism, and antioxidant contentin rat gastric mucosa.
Similar content being viewed by others
REFERENCES
Oates PJ, Hakkinen JP: Studies on the mechanism of ethanolinduced gastric damage in rats. Gastroenterology 94:10–21, 1988
Klaassen CHW, Swarts HGP, De Pont JJHHM: Ethanol stimulate s expression of functional H+, K+-ATPase in SF9 cells.Biochem Biophys Res Commun 210:907–913, 1995
Bell AE, Sellers LA, Allen A, Cunliffe WJ, Morris ER, Ross-Murphy SB: Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology 88:269–280, 1985
Nordman R, Ribiere C, Roach H: Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med 12:219–240, 1992
Gislason H, Herfjord JK, Guttu K, Grong K, Gronbech JE, Ulvik R, Svanes K: Adaptive protection related to clearance of ethanol in gastric mucosa of cats. Scand J Gastroenterol 28:361–368, 1993
Konturek SJ, Brzozowski T, Stachura J, Dembinski A, Majka J: Role of gastric blood flow, neutrophil infiltration and mucosal cell proliferation in gastric adaptation to aspirin in rats. Gut 35:1189–1196, 1994
Takeuchi K, Kato S, Yashuhiro T, Yagi K: Mechanism of acid secretory change s in rat stomach after damage by taurocholate. Dig Dis Sci 42:645–653, 1997
Takeuchi K, Okabe S: Mechanism of gastric alkaline response in the stomach after damage. Dig Dis Sci 40(4):865–871, 1995
Takeuchi K, Takehara K, Kaneko T, Okabe S: Nitric oxide and prostaglandins in regulation of acid secretory response in rat stomach following injury. J Pharmacol Exp Ther 272:357–363, 1995
Brown JF, Keates AC, Hanson PJ, Whittle BJR: Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol 265(Gastrointest Liver Physiol28):G418-G422, 1993
Kanwar S, Wallace JL, Befus D, Kubes P: Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol 266:G222-G229, 1994
Schmidt KL, Henagan JM, Mitchell PA, Smith GS, Miller TA: The protective effects of a prostaglandin without antisecretory properties against ethanol-induced injury in the rat stomach: A histologic study. Histol Histopathol 2:173–183, 1987
Nakao T, Tashima Y, Nagao K, et al: Highly specific sodiumpotassium-associated adenosine triphosphate from various tissues of rabbit. Biochem Biophys Res Commun. 19:755–758, 1965
Taussky HH, Shorr E: A micro colorimetric method for determination of inorganic phosphorus. J Biol Chem 202:675–685, 1953
Lowry OH, Rosebrough NJ, Farr AL, Randell RJ: Protein me asurement with Folin phenol reagent. J Biol Chem 193:265–275, 1951
Beauchamp C, Fridovich I: Superoxide dismutase: Improved assays and applicable to acrylamide gels. Anal Biochem 44:276–287, 1997
Arouma OI: Deoxyribose assay for detecting hydroxyl radicals. Methods Enzymol 233:57–66, 1994
Loguercio C, Taranto DE, Beneduce F, del Vecchio Blanco C, De Vincentiis A, Nardi G, Romano M: Glutathione prevents ethanol induced gastric mucosal damage and depletion of suflhydryl compounds in humans. Gut 34:161, 1993
Klaassen CHW, De Pont JJHHM: Gastric H+, K+-ATPase. Cell Physiol Biochem 4:115–134, 1994
Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S: Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Nattl Acad Sci USA 91(11):5212–5216, 1994
Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A: Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys Res Commun 214( 3):847–855, 1995
Julkunen RJK, Dipadove C, Lieber CS: First pass metabolism of ethanol— a gastrointestinal barrie r against the systemic toxicity of ethanol. Life Sci 37:567–573, 1985
Lee L, Schmidt KL, Tornwall MS, Henagan JM, Miller TA: Gender differences in ethanol oxidation and injury in the rat stomach. Alcohol 9:421–425, 1992
Ligumsky M, Sestieri M, Okon E, Ginsburg I: Antioxidants inhibit ethanol-induced gastric injury in the rat. Role of manganese, glycine, and carotene. Scand J Gastroenterol 30:854–860, 1995
Matsumoto T, Moriguchi R, Yamada H: Role of polymorphonuclear leucocytes and oxygen-derived free radicals in the formation of gastric lesions induced by HCl/ethanol, and a possible me chanism of protection by anti-ulcer polysaccharide. J Pharmacol 45:535–539, 1993
Goos SP, Hogg N, Kalyanaraman B: The antioxidant effect of spermine NONOate in human low density lipoprotein. Chem Res Toxicol 3:800–806, 1995
Grisham MB, von Ritten C, Smith BF, Lamont JT, Granger DN: Interaction between oxygen radicals and gastric mucin. Am J Physiol 253(Gastrointest Live r Physiol 16):G93-G96, 1987
Rights and permissions
About this article
Cite this article
Bulut, R., Unlucerci, Y., Bekpinar, S. et al. Nitric Oxide-Mediated Regulation of Gastric H+, K+-ATPase and Alcohol Dehydrogenase Following Ethanol-Induced Injury in Rats. Dig Dis Sci 44, 1417–1422 (1999). https://doi.org/10.1023/A:1026608020133
Issue Date:
DOI: https://doi.org/10.1023/A:1026608020133