Abstract
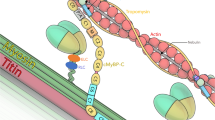
p0071 is a member of the armadillo gene family that is expressed in a wide variety of mammalian tissues and cell types with a prominent cell–cell contact association in epithelial cells. Here, we report the expression and localization patterns of p0071 in differentiating human skeletal muscle cells and in normal and diseased human skeletal muscle tissues. Northern blots revealed expression of p0071 mRNA in adult skeletal muscle tissue. RT-PCR analysis and Western blotting experiments identified two differentially spliced isoforms of p0071. The balance between these isoforms shifted during in vitro differentiation of isolated muscle cells from predominant expression of the short variant to a preponderance of the larger variant from day 6 onwards. Immunolocalization studies in mature skeletal muscle tissue revealed that p0071 is a constituent of myofibrils with a distinct localization at the level of sarcomeric N2-lines. During myofibrillogenesis, p0071 was not detected in non-striated nascent myofibrils, but became apparent shortly after the development of compact Z-discs in early myotubes. Furthermore, we studied the expression of p0071 in a wide variety of neuromuscular disorders by indirect immunofluorescence. Here, the myofibrillar staining of p0071 was preserved in all the disease entities included in our study. Our results provide the first evidence that a member of the armadillo multigene family is a constituent of the contractile apparatus in human skeletal muscle. The localization of p0071 at the level of I-bands and the timepoint of its integration into developing myofibrils suggest a possible role in the organization of thin filaments.
Similar content being viewed by others
References
Aberle H, Schwartz H and Kemler R (1996) Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 61: 514–523.
Behrens J (1999) Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev 18: 15–30.
Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R and Birchmeier W (1996) Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 382: 638–642.
Bierkamp C, McLaughlin KJ, Schwarz H, Huber O and Kemler R (1996) Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol 180: 780–785.
Bonne S, van Hengel J, Nollet F, Kools P and van Roy F (1999) Plakophilin-3, a novel Armadillo-like protein present in nuclei and desmosomes of epithelial cells. J Cell Sci 112: 2265–2276.
Bonne S, van Hengel J and van Roy F (1998) Chromosomal mapping of human armadillo genes belonging to the p120(ctn)/plakophilin subfamily. Genomics 51: 452–454.
Daniel JM and Reynolds AB (1999) The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol 19: 3614–3623.
Franke WW, Kapprell HP and Cowin P (1987) Plakoglobin is a component of the filamentous subplasmalemmal coat of lens cells. Eur J Cell Biol 43: 301–315.
Furst DO, Osborn M, Nave R and Weber K (1988) The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol 106: 1563–1572.
Gregorio CC (1997) Models of thin filament assembly in cardiac and skeletal muscle. Cell Struct Funct 22: 191–195.
Hatzfeld M (1999) The armadillo Family of Structural Proteins. Int Rev Cytol 186: 179–224.
Hatzfeld M, Kristjansson GI, Plessmann U and Weber K (1994) Band 6 protein, a major constituent of desmosomes from stratified epithelia, is a novel member of the armadillo multigene family. J Cell Sci 107: 2259–2270.
Hatzfeld M and Nachtsheim C (1996) Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: evidence for a subfamily of closely related proteins. J Cell Sci 109: 2767–2778.
Heid HW, Schmidt A, Zimbelmann R, Schafer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnolzer M and Franke WW (1994) Cell type-specific desmosomal plaque proteins of the plakoglobin family: plakophilin 1 (band 6 protein). Differentiation 58: 113–131.
Hertig CM, Butz S, Koch S, Eppenberger-Eberhardt M, Kemler R and Eppenberger HM (1996) N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. J Cell Sci 109: 11–20.
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG and Kemler R (1996) Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev 59: 3–10.
Kemler R (1993) From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet 9: 317–321.
Klymkowsky MW (1999) Plakophilin, armadillo repeats, and nuclear localization. Microsc Res Tech 45: 43–54.
Kowalczyk AP, Hatzfeld M, Bornslaeger EA, Kopp DS, Borgwardt JE, Corcoran CM, Settler A and Green KJ (1999) The head domain of plakophilin-1 binds to desmoplakin and enhances its recruitment to desmosomes. Implications for cutaneous disease. J Biol Chem 274: 18, 145–18, 148.
Laing NG (1999) Inherited disorders of sarcomeric proteins. Curr Opin Neurol 12: 513–518.
Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, Kneebone C, Blumbergs P, White S, Watkins H, Love DR et al. (1995) A mutation in the alpha tropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy NEM1. Nat Genet 10: 249.
Levesque G, Yu G, Nishimura M, Zhang DM, Levesque L, Yu H, Xu D, Liang Y, Rogaeva E, Ikeda M, Duthie M, Murgolo N, Wang L, Van der Vere P, Bayne ML, Strader CD, Rommens JM, Fraser PE and St. George-Hyslop P (1999) Presenilins interact with armadillo proteins including neural-specific plakophilin-related protein and beta-catenin. J Neurochem 72: 999–1008.
Linask KK, Knudsen KA and Gui YH (1997) N-cadherin-catenin interaction: necessary component of cardiac cell compartmentalization during early vertebrate heart development. Dev Biol 185: 148–164.
Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L and Kosik KS (1999) delta-catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol 144: 519–532.
Mariner DJ, Sirotkin H, Daniel JM, Lindman BR, Mernaugh RL, Patten AK, Thoreson MA and Reynolds AB (1999) Production and characterization of monoclonal antibodies to ARVCF. Hybridoma 18: 343–349.
Mariner DJ, Wang J and Reynolds AB (2000) ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120(ctn) in E-cadherin complexes. J Cell Sci 113: 1481–1490.
Marrs JA and Nelson WJ (1996) Cadherin cell adhesion molecules in differentiation and embryogenesis. Int Rev Cytol 165: 159–205.
Maruyama K, Endo T, Kume H, Kawamura Y, Kanzawa N, Nakauchi Y, Kimura S and Kawashima S (1993) A novel domain sequence of connectin localized at the I band of skeletal muscle sarcomeres: homology to neurofilament subunits. Biochem Biophys Res Commun 194: 1288–1291.
McCrea PD, Turck CW and Gumbiner B (1991) A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254: 1359–1361.
Mertens C, Kuhn C and Franke WW (1996) Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135: 1009–1025.
Mertens C, Kuhn C, Moll R, Schwetlick I and Franke WW (1999) Desmosomal plakophilin 2 as a differentiation marker in normal and malignant tissues. Differentiation 64: 277–290.
Newman GR, Jasani B and Williams ED (1983) A simple post-embedding system for the rapid demonstration of tissue antigens under the electron microscope. Histochem J 15: 543–555.
Nowak KJ, Wattanasirichaigoon D, Goebel HH, Wilce M, Pelin K, Donner K, Jacob RL, Hubner C, Oexle K, Anderson JR, Verity CM, North KN, Iannaccone ST, Muller CR, Nurnberg P, Muntoni F, Sewry C, Hughes I, Sutphen R, Lacson AG, Swoboda KJ, Vigneron J, Wallgren-Pettersson C, Beggs AH and Laing NG (1999) Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet 23: 208–212.
Paffenholz R and Franke WW (1997) Identification and localization of a neurally expressed member of the plakoglobin/armadillo multigene family. Differentiation 61: 293–304.
Peifer M, Berg S and Reynolds AB (1994) A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76: 789–791.
Peifer M, McCrea PD, Green KJ, Wieschaus E and Gumbiner BM (1992) The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol 118: 681–691.
Peifer M, Orsulic S, Pai LM and Loureiro J (1993) A model system for cell adhesion and signal transduction in Drosophila. Dev Suppl 1: 63–76.
Peifer M and Wieschaus E (1990) The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell 63: 1167–1176.
Pelin K, Hilpela P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea JA, Muntoni F, Dubowitz V, Beggs AH, Laing NG, Labeit S, de la Chapelle A and Wallgren-Pettersson C (1999) Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 96: 2305–2310.
Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J and Zhang Z (1994) Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14: 8333–8342.
Reynolds AB and Daniel JM (1997) p120ctn: a src-substrate turned catenin. In: Cowin P, Klymkowsky MW (eds.) Cytoskeletal-Membrane Interactions and Signal Transduction, (pp. 31–48), Landes Bioscience, Austin, TX, USA.
Reynolds AB, Herbert L, Cleveland JL, Berg ST and Gaut JR (1992) p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene 7: 2439–2445.
Rhee D, Sanger JM and Sanger JW (1994) The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton 28: 1–24.
Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke WW and Birchmeier W (1996) Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol 135: 215–225.
Sanger JW, Mittal B and Sanger JM (1984) Formation of myofibrils in spreading chick cardiac myocytes. Cell Motil 4: 405–416.
Schmidt A, Langbein L, Pratzel S, Rode M, Rackwitz HR and Franke WW (1999) Plakophilin 3-a novel cell-type-specific desmosomal plaque protein. Differentiation 64: 291–306.
Schmidt A, Langbein L, Rode M, Prätzel S, Zimbelmann R and Franke WW (1997) Plakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res 290: 481–499.
Schröder R, Warlo I, Herrmann H, van der Ven PF, Klasen C, Blumcke I, Mundegar RR, Furst DO, Goebel HH and Magin TM (1999) Immunogold EM reveals a close association of plectin and the desmin cytoskeleton in human skeletal muscle. Eur J Cell Biol 78: 288–295.
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 96: 5522–5527.
Sirotkin H, H OD, DasGupta R, Halford S, St. Jore B, Puech A, Parimoo S, Morrow B, Skoultchi A, Weissman SM, Scambler P and Kucherlapati R (1997) Identification of a new human catenin gene family member (ARVCF) from the region deleted in velo-cardio-facial syndrome. Genomics 41: 75–83.
Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K et al. (1995) Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem 270: 31, 158–31, 162.
Stahl B, Diehlmann A and Sudhof TC (1999) Direct interaction of Alzheimer's disease-related presenilin 1 with armadillo protein p0071. J Biol Chem 274: 9141–9148.
Tanahashi H and Tabira T (1999) Isolation of human delta-catenin and its binding specificity with presenilin 1. Neuroreport 10: 563–568.
Tetsu O and McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426.
Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK and Reynolds AB (2000) Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol 148: 189–202.
van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M and Clevers H (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799.
van der Ven PF and Furst DO (1997) Assembly of titin, myomesin and M-protein into the sarcomeric M band in differentiating human skeletal muscle cells in vitro. Cell Struct Funct 22: 163–171.
van der Ven PF, Schaart G, Croes HJ, Jap PH, Ginsel LA and Ramaekers FC (1993) Titin aggregates associated with intermediate filaments align along stress fiber-like structures during human skeletal muscle cell differentiation. J Cell Sci 106: 749–759.
van Hengel J, Vanhoenacker P, Staes K and van Roy F (1999) Nuclear localization of the p120(ctn) armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc Natl Acad Sci USA 96: 7980–7985.
Wallgren-Pettersson C, Pelin K, Hilpela P, Donner K, Porfirio B, Graziano C, Swoboda KJ, Fardeau M, Urtizberea JA, Muntoni F, Sewry C, Dubowitz V, Iannaccone S, Minetti C, Pedemonte M, Seri M, Cusano R, Lammens M, Castagna-Sloane A, Beggs AH, Laing NG and de la Chapelle A (1999) Clinical and genetic heterogeneity in autosomal recessive nemaline myopathy. Neuromuscul Disord 9: 564–572.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schröder, R., Van der Ven, P.F.M., Warlo, I. et al. p0071, a member of the armadillo multigene family, is a constituent of sarcomeric I-bands in human skeletal muscle. J Muscle Res Cell Motil 21, 577–586 (2000). https://doi.org/10.1023/A:1026587530656
Issue Date:
DOI: https://doi.org/10.1023/A:1026587530656